7 Calvin C. Sheng1, Jijun Hao2, and Charles C. Hong3 1Department of Cardiovascular Medicine, Vanderbilt University, USA 2College of Veterinary Medicine, Western University of Health Sciences, USA 3Department of Medicine, Vanderbilt University, USA The concept of “stem cells” first emerged in the 1960s when transplanted mouse marrow-derived cell colonies were shown to have the ability to differentiate into three hematologic lineages: erythrocytes, granulocytes, and megakaryocytes [1]. After this, researchers began searching for the progenitor cells responsible for generating all other cell types during embryonic development. It was not until the early 1980s that mouse embryonic stem cells (mESCs) were first successfully isolated and established as in vitro cultures [2,3]. In the past 3 decades, newer platforms, including human embryonic stem cells (hESCs) [4], human induced pluripotent stem cells (hiPSCs) [5], and, most recently, chemically induced pluripotent stem cells (CiPSCs) [6], have been developed as tools for disease modeling, drug discovery, and regenerative medicine. Each platform provided a promising glimpse of what the revolutionizing field of stem-cell research had to offer in terms of therapeutics and translational medicine. However, they all share a common set of technical challenges: how to develop a renewable and efficient source of pluripotent cells, maintain a stable population of pluripotent cells in the undifferentiated state without accumulating genetic abnormalities, and guide differentiation to yield a homogenous somatic cell population. In this chapter, we will focus on a discussion of CiPSCs, the most recent technology (reported in July 2013), and their advantages, disadvantages, and application in regenerative medicine [6]. Pluripotent stem cells can be derived through either direct harvesting of embryonic stem cells (ESCs) or, more recently, reprogramming of somatic cells. The latter was first achieved by Yamanaka’s group using a cocktail of four transcription factors (Oct3/4, Sox2, Klf4, and c-Myc) [5, 7], circumventing the ethical dilemma of destroying the fertilized embryos associated with ESCs and providing an autologous source of stem cells less prone to immune rejection after transplant. However, hiPSCs must still overcome several limitations, such as low cellular reprogramming efficiency, incomplete reprogramming resulting in heterogeneous population of cells, and risk of tumorigenesis [8]. In particular, c-Myc and Klf4 are known tumorigenic proteins, whose interactions with p53 lead to genomic instability. Within the last year, one study has reported successful reprogramming of murine somatic cells into CiPSCs using only small molecules [6]. This replacement of transcription factors with small molecules offers several distinct advantages during traditional reprogramming and differentiation methods (Table 7.1). First, small molecules are much more cost-effective than biological reagents, and exhibit consistent potency and stability [9]. Second, small molecules can act as reversible modulators, allowing for temporal regulation with high precision [10]. Genetic approaches, by contrast, involve permanent genome modifications with attendant problems of tumorigenicity and other irreversible, unintended consequences. Temporal regulation is also an issue with biological reagents in regards to the duration of effects in in vivo models. Even newer reagents, such as small interfering RNAs (siRNAs), typically function as genetic switches in an all-or-none manner, leaving little room for procedural fine-tuning. The final and most crucial point, which is especially germane to the reprogramming efforts, is that cell-permeable small molecules have the potential to target every class of macromolecule in the cell. By contrast, biological reagents are best suited to targeting extracellular components. While extracellular signals invariably influence pluripotency, modulation of extracellular signals alone is insufficient to regulate reprogramming events in the nucleus. Table 7.1 Comparison of available stem-cell technologies One of the main drawbacks to using small molecules is that our current database of bioactive compounds and their cellular effects is far from comprehensive. We do not have a complete database of validated compounds that specifically target every cellular component and pathway. Furthermore, because many small molecules are not truly specific and may have multiple targets, it is more difficult to define a precise mechanism of action. Another concern is that some of the chemicals used in the reprogramming process directly impact the cells at the epigenetic level, leading to potential chemically induced mutations [11]. For instance, BayK (a specific L-type calcium channel agonist) in the presence of BIX (a G9a methyltransferase inhibitor) successfully replaced Sox2 and c-Myc during reprogramming of mouse embryonic fibroblasts, but BIX as a key regulator of DNA methylation and transcriptional silencing may concomitantly reactivate undesirable oncogenic genes (Table 7.2) [12]. Table 7.2 Small molecules shown to have enhanced reprogramming efficiency
Chemically Induced Pluripotent Stem Cells (CiPSCs): A Potential Chemical Biological Breakthrough in Reprogramming?
7.1 Searching for the “Perfect” Platform
7.2 Defining the Advantages of Small Molecules in Reprogramming
Safe,
Established
Established
Autologous
Easy to
robust, and
Disease
Regenerative
mouse line
human line
cells
obtain
cost-effective
modeling
medicine
CiPSCs
✓
×
✓
✓
?
?
?
iPSCs
✓
✓
✓
✓
?
✓
?
ESCs
✓
✓
×
×
×
✓
?
7.3 Understanding the Disadvantages of Using Small Molecules
Small-molecule compound
Chemical structure
Mechanism of action
BayK8644 [12]
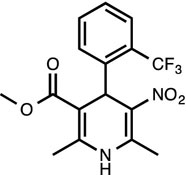
L-type calcium channel agonist
BIX01294 [24]
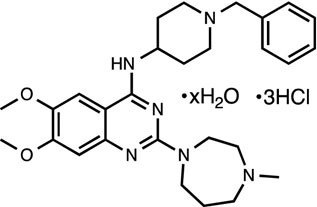
G9a methytransferase inhibitor
SB431542 [8]
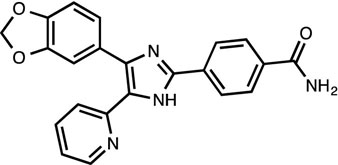
TGFβ inhibitor
PD0325901 [8, 19]
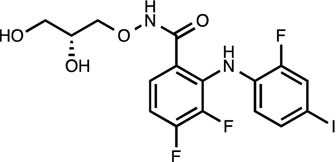
MEK inhibitor
Thiazovivin [8]
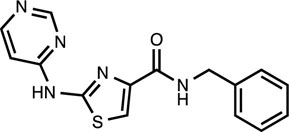
ROCK inhibitor ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
