Figure 1-1. General structure of a neuron. This is an artist’s conception of the generic structure of a neuron. All neurons have a cell body known as the soma, which is the command center of the nerve and contains the nucleus of the cell. All neurons are also set up structurally to both send and receive information. Neurons send information via an axon that forms presynaptic terminals as the axon passes by (en passant) or as the axon ends.
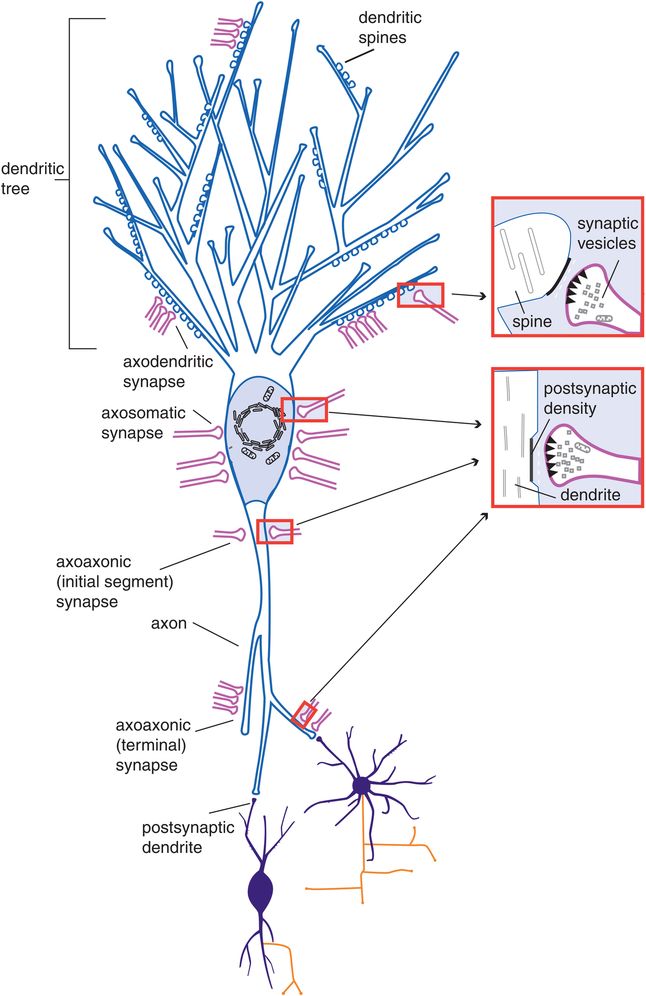
Figure 1-2. Axodendritic, axosomatic, and axoaxonic connections. After neurons migrate, they form synapses. As shown in this figure, synaptic connections can form not just between the axon and dendrites of two neurons (axodendritic) but also between the axon and the soma (axosomatic) or the axons of the two neurons (axoaxonic). Communication is anterograde from the axon of the first neuron to the dendrite, soma, or axon of the second neuron.
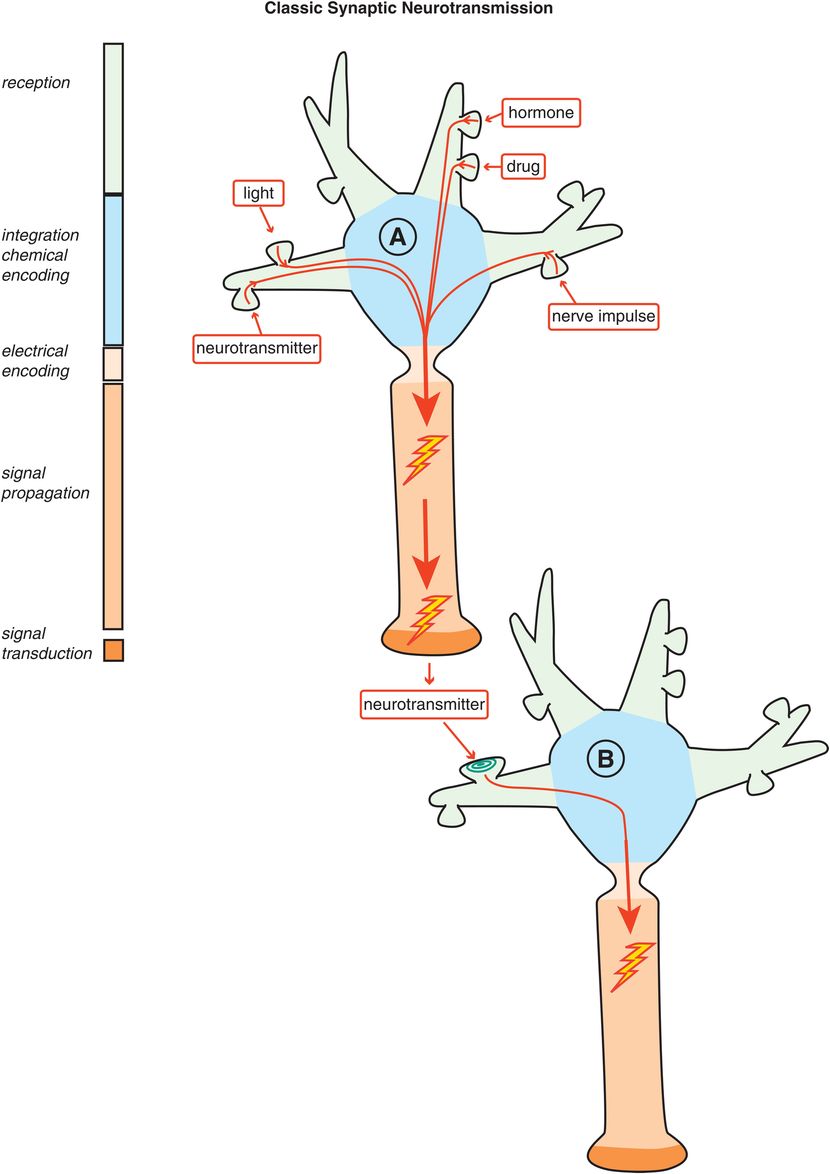
Figure 1-3. Classic synaptic neurotransmission. In classic synaptic neurotransmission, stimulation of a presynaptic neuron (e.g., by neurotransmitters, light, drugs, hormones, nerve impulses) causes electrical impulses to be sent to its axon terminal. These electrical impulses are then converted into chemical messengers and released to stimulate the receptors of a postsynaptic neuron. Thus, although communication within a neuron can be electrical, communication between neurons is chemical.
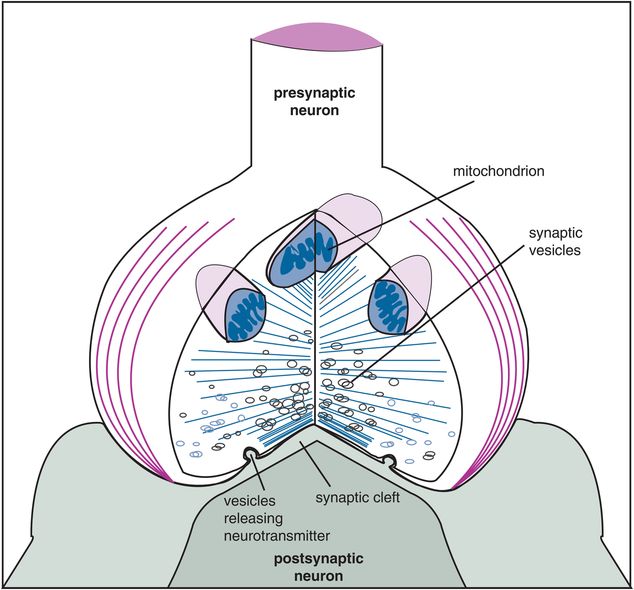
Figure 1-4. Enlarged synapse. The synapse is enlarged conceptually here to show the specialized structures that enable chemical neurotransmission to occur. Specifically, a presynaptic neuron sends its axon terminal to form a synapse with a postsynaptic neuron. Energy for neurotransmission from the presynaptic neuron is provided by mitochondria there. Chemical neurotransmitters are stored in small vesicles, ready for release upon firing of the presynaptic neuron. The synaptic cleft is the gap between the presynaptic neuron and the postsynaptic neuron; it contains proteins and scaffolding and molecular forms of “synaptic glue” to reinforce the connection between the neurons. Receptors are present on both sides of this cleft and are key elements of chemical neurotransmission.
Neurons are the cells of chemical communication in the brain. Human brains are comprised of tens of billions of neurons, and each is linked to thousands of other neurons. Thus, the brain has trillions of specialized connections known as synapses. Neurons have many sizes, lengths, and shapes that determine their functions. Localization within the brain also determines function. When neurons malfunction, behavioral symptoms may occur. When drugs alter neuronal function, behavioral symptoms may be relieved, worsened, or produced.
General structure of a neuron. Although this textbook will often portray neurons with a generic structure (such as that shown in Figures 1-1 through 1-3), the truth is that many neurons have unique structures depending upon where in the brain they are located and what their function is. All neurons have a cell body known as the soma, and are set up structurally to receive information from other neurons through dendrites, sometimes via spines on the dendrites and often through an elaborately branching “tree” of dendrites (Figure 1-2). Neurons are also set up structurally to send information to other neurons via an axon that forms presynaptic terminals as the axon passes by (en passant, Figure 1-1) or as the axon ends (presynaptic axon terminals, Figures 1-1 through 1-4).
Neurotransmission has an anatomical infrastructure, but it is fundamentally a very elegant chemical operation. Complementary to the anatomically addressed nervous system is the chemically addressed nervous system, which forms the chemical basis of neurotransmission: namely, how chemical signals are coded, decoded, transduced, and sent along the way. Understanding the principles of chemical neurotransmission is a fundamental requirement for grasping how psychopharmacologic agents work, because they target key molecules involved in neurotransmission. Drug targeting of specific chemical sites that influence neurotransmission is discussed in Chapters 2 and 3.
Understanding the chemically addressed nervous system is also a prerequisite for becoming a “neurobiologically informed” clinician: that is, being able to translate exciting new findings on brain circuitry, functional neuroimaging, and genetics into clinical practice, and potentially improving the manner in which psychiatric disorders and their symptoms are diagnosed and treated. The chemistry of neurotransmission in specific brain regions and how these principles are applied to various specific psychiatric disorders and treated with various specific psychotropic drugs are discussed throughout the rest of the book.
Principles of chemical neurotransmission
Neurotransmitters
There are more than a dozen known or suspected neurotransmitters in the brain. For psychopharmacologists, it is particularly important to know the six key neurotransmitter systems targeted by psychotropic drugs:
- serotonin
- norepinephrine
- dopamine
- acetylcholine
- glutamate
- GABA (γ-aminobutyric acid)
Each is discussed in detail in the clinical chapters related to the specific drugs that target them. Other neurotransmitters that are also important neurotransmitters and neuromodulators, such as histamine and various neuropeptides and hormones, are mentioned in brief throughout the relevant clinical chapters in this textbook.
Some neurotransmitters are very similar to drugs and have been called “God’s pharmacopeia.” For example, it is well known that the brain makes its own morphine (i.e., β-endorphin) and its own marijuana (i.e., anandamide). The brain may even make its own antidepressants, anxiolytics, and hallucinogens. Drugs often mimic the brain’s natural neurotransmitters, and some drugs have been discovered prior to the natural neurotransmitter. Thus, morphine was used in clinical practice before the discovery of β-endorphin; marijuana was smoked before the discovery of cannabinoid receptors and anandamide; the benzodiazepines Valium (diazepam) and Xanax (alprazolam) were prescribed before the discovery of benzodiazepine receptors; and the antidepressants Elavil (amitriptyline) and Prozac (fluoxetine) entered clinical practice before molecular clarification of the serotonin transporter site. This underscores the point that the great majority of drugs that act in the central nervous system act upon the process of neurotransmission. Indeed, this apparently occurs at times in a manner that can mimic the actions of the brain itself, when the brain uses its own chemicals.
Input to any neuron can involve many different neurotransmitters coming from many different neuronal circuits. Understanding these inputs to neurons within functioning circuits can provide a rational basis for selecting and combining therapeutic agents. This theme is discussed extensively in each chapter on the various psychiatric disorders. The idea is that for the modern psychopharmacologist to influence abnormal neurotransmission in patients with psychiatric disorders, it may be necessary to target neurons in specific circuits. Since these networks of neurons send and receive information via a variety of neurotransmitters, it may therefore be not only rational but necessary to use multiple drugs with multiple neurotransmitter actions for patients with psychiatric disorders, especially if single agents with single neurotransmitter mechanisms are not effective in relieving symptoms.
Neurotransmission: classic, retrograde, and volume
Classic neurotransmission begins with an electrical process by which neurons send electrical impulses from one part of the cell to another part of the same cell via their axons (see neuron A in Figure 1-3). However, these electrical impulses do not jump directly to other neurons. Classic neurotransmission between neurons involves one neuron hurling a chemical messenger, or neurotransmitter, at the receptors of a second neuron (see the synapse between neuron A and neuron B in Figure 1-3). This happens frequently but not exclusively at the sites of synaptic connections. In the human brain, a hundred billion neurons each make thousands of synapses with other neurons for an estimated trillion chemically neurotransmitting synapses.
Communication between all these neurons at synapses is chemical, not electrical. That is, an electrical impulse in the first neuron is converted to a chemical signal at the synapse between it and a second neuron, in a process known as excitation–secretion coupling, the first stage of chemical neurotransmission. This occurs predominantly but not exclusively in one direction, from the presynaptic axon terminal to a second postsynaptic neuron (Figures 1-2 and 1-3). Finally, neurotransmission continues in the second neuron either by converting the chemical information from the first neuron back into an electrical impulse in the second neuron, or, perhaps more elegantly, by the chemical information from the first neuron triggering a cascade of further chemical messages within the second neuron to change that neuron’s molecular and genetic functioning (Figure 1-3).
An interesting twist to chemical neurotransmission is the discovery that postsynaptic neurons can also “talk back” to their presynaptic neurons. They can do this via retrograde neurotransmission from the second neuron to the first at the synapse between them (Figure 1-5, right panel). Chemicals produced specifically as retrograde neurotransmitters at some synapses include the endocannabinoids (EC, also known as “endogenous marijuana”), which are synthesized in the postsynaptic neuron. They are then released and diffuse to presynaptic cannabinoid receptors such as the CB1 or cannabinoid 1 receptor (Figure 1-5, right panel). Another retrograde neurotransmitter is the gaseous neurotransmitter NO, or nitric oxide, which is synthesized postsynaptically and then diffuses out of the postsynaptic membrane and into the presynaptic membrane to interact with cyclic guanosine monophosphate (cGMP)-sensitive targets there (Figure 1-5, right panel). A third group of retrograde neurotransmitter are neurotrophic factors such as NGF (nerve growth factor), which is released from postsynaptic sites and then diffuses to the presynaptic neuron, where it is taken up into vesicles and transported all the way back to the cell nucleus via retrograde transport systems to interact with the genome there (Figure 1-5, right panel). What these retrograde neurotransmitters have to say to the presynaptic neuron and how this modifies or regulates the communication between pre- and postsynaptic neuron are subjects of intense active investigation.

Figure 1-5. Retrograde neurotransmission. Not all neurotransmission is classic or anterograde or from top to bottom – namely, presynaptic to postsynaptic (left). Postsynaptic neurons may also communicate with presynaptic neurons from the bottom to the top via retrograde neurotransmission, from postsynaptic neuron to presynaptic neuron (right). Some neurotransmitters produced specifically as retrograde neurotransmitters at some synapses include the endocannabinoids (ECs, or “endogenous marijuana”), which are synthesized in the postsynaptic neuron, released, and diffuse to presynaptic cannabinoid receptors such as the cannabinoid 1 receptor (CB1); the gaseous neurotransmitter nitric oxide (NO), which is synthesized postsynaptically and then diffuses both out of the postsynaptic membrane and into the presynaptic membrane to interact with cyclic guanosine monophosphate (cGMP)-sensitive targets there; and neurotrophic factors such as nerve growth factor (NGF), which is released from postsynaptic sites and diffuses to the presynaptic neuron, where it is taken up into vesicles and transported all the way back to the cell nucleus via retrograde transport systems to interact with the genome there.
In addition to “reverse” or retrograde neurotransmission at synapses, some neurotransmission does not need a synapse at all! Neurotransmission without a synapse is called volume neurotransmission, or nonsynaptic diffusion neurotransmission (examples are shown in Figures 1-6 through 1-8). Chemical messengers sent by one neuron to another can spill over to sites distant to the synapse by diffusion (Figure 1-6). Thus, neurotransmission can occur at any compatible receptor within the diffusion radius of the neurotransmitter, not unlike modern communication with cellular telephones, which function within the transmitting radius of a given cell tower (Figure 1-6). This concept is part of the chemically addressed nervous system, and here neurotransmission occurs in chemical “puffs” (Figures 1-6 through 1-8). The brain is thus not only a collection of wires, but also a sophisticated “chemical soup.” The chemically addressed nervous system is particularly important in mediating the actions of drugs that act at various neurotransmitter receptors, since such drugs will act wherever there are relevant receptors, and not just where such receptors are innervated with synapses by the anatomically addressed nervous system. Modifying volume neurotransmission may indeed be a major way in which several psychotropic drugs work in the brain.
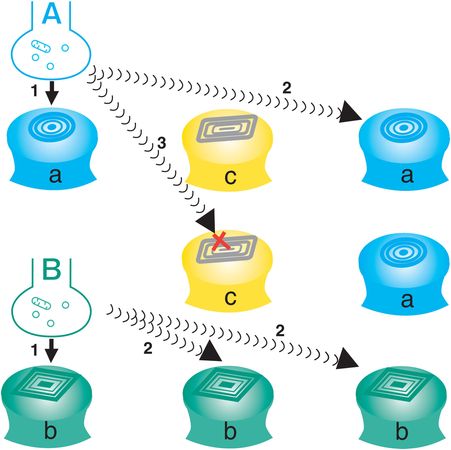
Figure 1-6. Volume neurotransmission. Neurotransmission can also occur without a synapse; this is called volume neurotransmission or nonsynaptic diffusion. In this figure, two anatomically addressed synapses (neurons A and B) are shown communicating with their corresponding postsynaptic receptors (a and b, arrows 1). However, there are also receptors for neurotransmitter A, neurotransmitter B, and neurotransmitter C, which are distant from the synaptic connections of the anatomically addressed nervous system. If neurotransmitter A or B can diffuse away from its synapse before it is destroyed, it will be able to interact with other matching receptor sites distant from its own synapse (arrows 2). If neurotransmitter A or B encounters a different receptor not capable of recognizing it (receptor c), it will not interact with that receptor even if it diffuses there (arrow 3). Thus, a chemical messenger sent by one neuron to another can spill over by diffusion to sites distant from its own synapse. Neurotransmission can occur at a compatible receptor within the diffusion radius of the matched neurotransmitter. This is analogous to modern communication with cellular telephones, which function within the transmitting radius of a given cell. This concept is called the chemically addressed nervous system, in which neurotransmission occurs in chemical “puffs.” The brain is thus not only a collection of wires but also a sophisticated “chemical soup.”
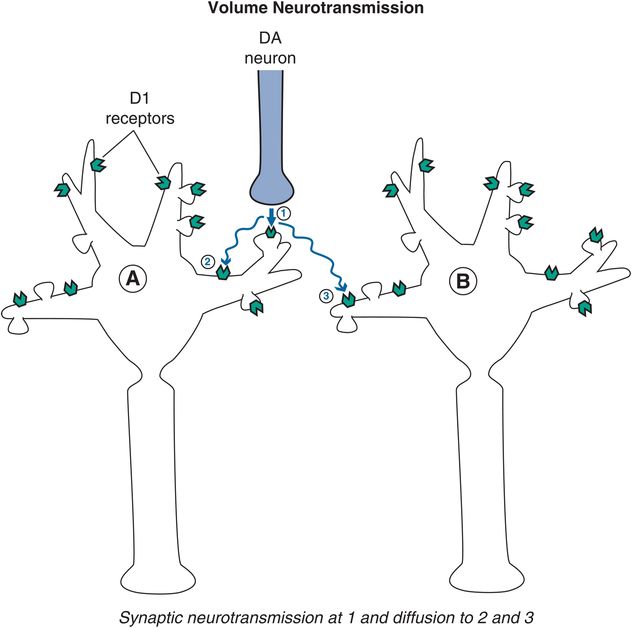
Figure 1-7. Volume neurotransmission: dopamine. An example of volume neurotransmission would be that of dopamine in the prefrontal cortex. Since there are few dopamine reuptake pumps in the prefrontal cortex, dopamine is available to diffuse to nearby receptor sites. Thus, dopamine released from a synapse (arrow 1) targeting postsynaptic neuron A is free to diffuse further in the absence of a reuptake pump and can reach dopamine receptors on that same neuron but outside of the synapse from which it was released, on neighboring dendrites (arrow 2). Shown here is dopamine also reaching extrasynaptic receptors on a neighboring neuron (arrow 3).
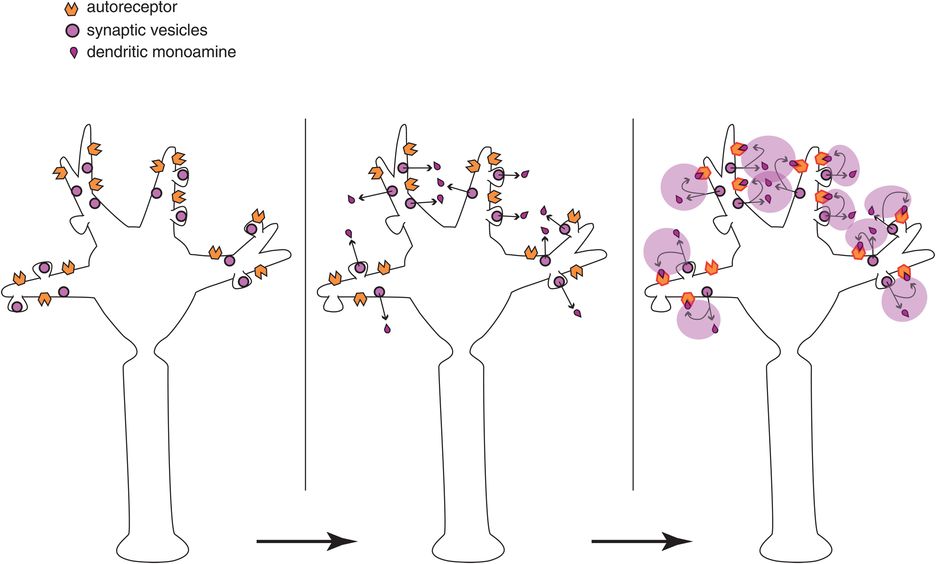
Figure 1-8. Volume neurotransmission: monoamine autoreceptors. Another example of volume neurotransmission could involve autoreceptors on monoamine neurons. Autoreceptors located on the dendrites and soma of a neuron (at the top of the neuron in the left panel) normally inhibit release of neurotransmitter from the axon of that neuron (at the bottom of the neuron in the left panel), and thus inhibit impulse flow through that neuron from top to bottom. Monoamines released from the dendrites of this neuron (at the top of the neuron in the middle panel), then bind to these autoreceptors (at the top of the neuron in the right panel) and would inhibit neuronal impulse flow in that neuron (from the bottom of the neuron in the right panel). This action occurs due to volume neurotransmission and despite the absence of synaptic neurotransmission in the somatodendritic areas of these neurons.
A good example of volume neurotransmission is dopamine action in the prefrontal cortex. Here there are very few dopamine reuptake transport pumps (dopamine transporters or DATs) to terminate the action of dopamine released in the prefrontal cortex during neurotransmission. This is much different from other brain areas, such as the striatum, where dopamine reuptake pumps are present in abundance. Thus, when dopamine neurotransmission occurs at a synapse in the prefrontal cortex, dopamine is free to spill over from that synapse and diffuse to neighboring dopamine receptors to stimulate them, even though there is no synapse at these “spillover” sites (Figure 1-7).
Another important example of volume neurotransmission is at the sites of autoreceptors on monoamine neurons (Figure 1-8). At the somatodendritic end of the neuron (top of the neurons in Figure 1-8) are autoreceptors that inhibit the release of neurotransmitter from the axonal end of the neuron (bottom of the neurons in Figure 1-8). Although some recurrent axon collaterals and other monoamine neurons may directly innervate somatodendritic receptors, these so-called somatodendritic autoreceptors also receive neurotransmitter from dendritic release (Figure 1-8, middle and right panels). There is no synapse here, just neurotransmitter leaked from the neuron upon its own receptors. The nature of a neuron’s regulation by its somatodendritic autoreceptors is a subject of intense interest, and is theoretically linked to the mechanism of action of many antidepressants, as will be explained in Chapter 7. The take-home point here is that not all chemical neurotransmission occurs at synapses.
Excitation–secretion coupling
An electrical impulse in the first – or presynaptic – neuron is converted into a chemical signal at the synapse by a process known as excitation–secretion coupling. Once an electrical impulse invades the presynaptic axon terminal, it causes the release of chemical neurotransmitter stored there (Figures 1-3 and 1-4). Electrical impulses open ion channels – both voltage-sensitive sodium channels (VSSCs) and voltage-sensitive calcium channels (VSCCs) – by changing the ionic charge across neuronal membranes. As sodium flows into the presynaptic nerve through sodium channels in the axon membrane, the electrical charge of the action potential moves along the axon until it reaches the presynaptic nerve terminal, where it also opens calcium channels. As calcium flows into the presynaptic nerve terminal, it causes synaptic vesicles anchored to the inner membrane to spill their chemical contents into the synapse. The way is paved for chemical communication by previous synthesis of neurotransmitter and storage of neurotransmitter in the first neuron’s presynaptic axon terminal.
Excitation–secretion coupling is thus the way that the neuron transduces an electrical stimulus into a chemical event. This happens very quickly once the electrical impulse enters the presynaptic neuron. It is also possible for the neuron to transduce a chemical message from a presynaptic neuron back into an electrical chemical message in the postsynaptic neuron by opening ion channels linked to neurotransmitters there. This also happens very quickly when chemical neurotransmitters open ion channels that change the flow of charge into the neuron, and ultimately, action potentials in the postsynaptic neuron. Thus, the process of neurotransmission is constantly transducing chemical signals into electrical signals, and electrical signals back into chemical signals.
Signal transduction cascades
Overview
Neurotransmission can be seen as part of a much larger process than just the communication of a presynaptic axon with a postsynaptic neuron at the synapse between them. That is, neurotransmission can also be seen as communication from the genome of the presynaptic neuron (neuron A in Figure 1-3) to the genome of the postsynaptic neuron (neuron B in Figure 1-3), and then back from the genome of the postsynaptic neuron to the genome of the presynaptic neuron via retrograde neurotransmission (right panel in Figure 1-5). Such a process involves long strings of chemical messages within both presynaptic and postsynaptic neurons, called signal transduction cascades.
Signal transduction cascades triggered by chemical neurotransmission thus involve numerous molecules, starting with neurotransmitter first messenger, and proceeding to second, third, fourth, and more messengers (Figures 1-9 through 1-30). The initial events occur in less than a second, but the long-term consequences are mediated by downstream messengers that take hours to days to activate, yet can last for many days or even for the lifetime of a synapse or neuron (Figure 1-10). Signal transduction cascades are somewhat akin to a molecular “pony express” with specialized molecules acting as a sequence of riders, handing on the message to the next specialized molecule, until the message has reached a functional destination, such as gene expression or activation of otherwise “sleeping” and inactive molecules (see for example, Figures 1-9 through 1-19).
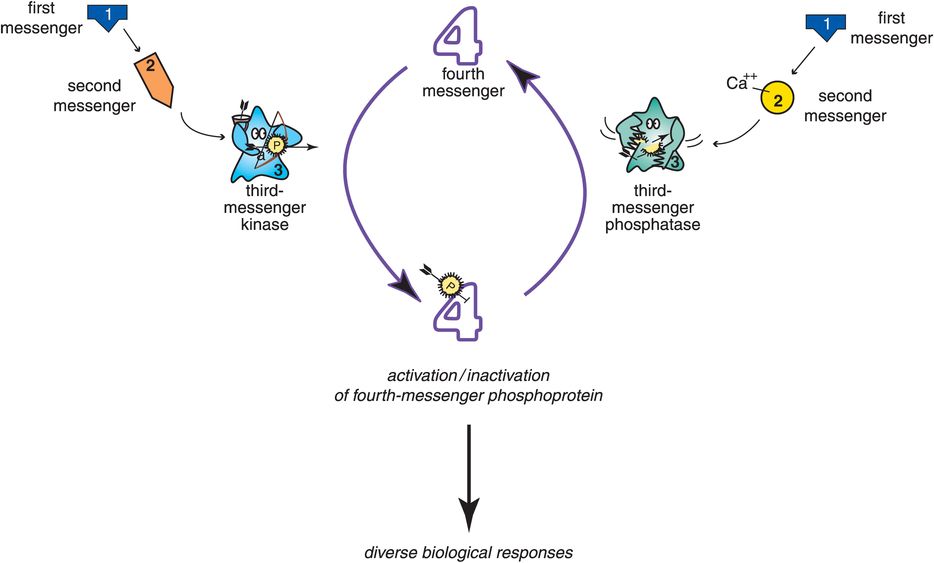
Figure 1-9. Signal transduction cascade. The cascade of events that occurs following stimulation of a postsynaptic receptor is known as signal transduction. Signal transduction cascades can activate third-messenger enzymes known as kinases, which add phosphate groups to proteins to create phosphoproteins (on the left). Other signal transduction cascades can activate third-messenger enzymes known as phosphatases, which remove phosphates from phosphoproteins (on the right). The balance between kinase and phosphatase activity, signaled by the balance between the two neurotransmitters that activate each of them, determines the degree of downstream chemical activity that gets translated into diverse biological responses, such as gene expression and synaptogenesis.
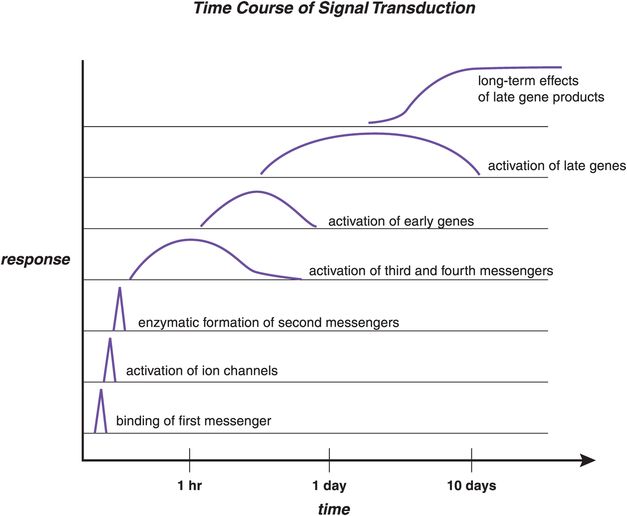
Figure 1-10. Time course of signal transduction. The time course of signal transduction is shown here. The process begins with binding of a first messenger (bottom), which leads to activation of ion channels or enzymatic formation of second messengers. This, in turn, can cause activation of third and fourth messengers, which are often phosphoproteins. If genes are subsequently activated, this leads to the synthesis of new proteins, which can alter the neuron’s functions. Once initiated, the functional changes due to protein activation or new protein synthesis can last for at least many days and possibly much longer. Thus, the ultimate effects of signal transduction cascades triggered by chemical neurotransmission are not only delayed but also long-lasting.
An overview of such a molecular “pony express,” from first-messenger neurotransmitter through several “molecular riders” to the production of diverse biological responses, is shown in Figure 1-9. Specifically, a first-messenger neurotransmitter on the left activates the production of a chemical second messenger that in turn activates a third messenger, namely an enzyme known as a kinase that adds phosphate groups to fourth-messenger proteins to create phosphoproteins (Figure 1-9, left). Another signal transduction cascade is shown on the right with a first-messenger neurotransmitter opening an ion channel that allows calcium to enter the neuron and act as the second messenger for this cascade system (Figure 1-9, right). Calcium then activates a different third messenger, namely an enzyme known as a phosphatase that removes phosphate groups from fourth-messenger phosphoproteins and thus reverses the actions of the third messenger on the left. The balance between kinase and phosphatase activity, signaled by the balance between the two neurotransmitters that activate each of them, determines the degree of downstream chemical activity that gets translated into active fourth messengers able to trigger diverse biological responses, such as gene expression and synaptogenesis (Figure 1-9). Each molecular site within the transduction cascade of chemical and electrical messages is a potential location for a malfunction associated with a mental illness; it is also a potential target for a psychotropic drug. Thus, the various elements of multiple signal transduction cascades play very important roles in psychopharmacology.
Four of the most important signal transduction cascades in the brain are shown in Figure 1-11. These include G-protein-linked systems, ion-channel-linked systems, hormone-linked systems, and neurotrophin-linked systems. There are many chemical messengers for each of these four critical signal transduction cascades; the G-protein-linked and the ion-channel-linked cascades are triggered by neurotransmitters (Figure 1-11). Many of the psychotropic drugs used in clinical practice today target one of these two signal transduction cascades. Drugs that target the G-protein-linked system are discussed in Chapter 2; drugs that target the ion-channel-linked system are discussed in Chapter 3.
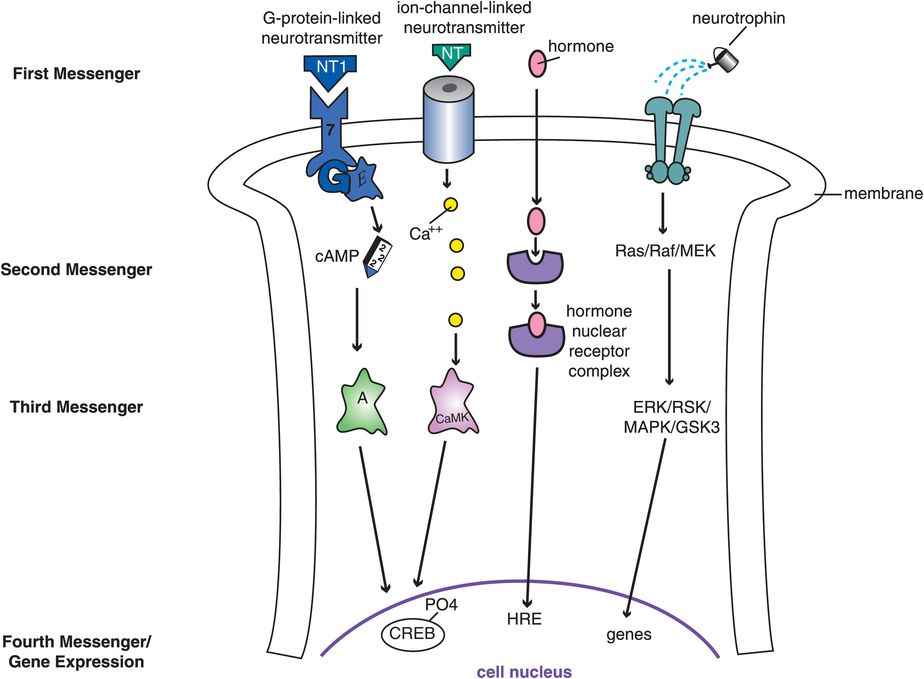
Figure 1-11. Different signal transduction cascades. Four of the most important signal transduction cascades in the brain are shown here. These include G-protein-linked systems, ion-channel-linked systems, hormone-linked systems, and neurotrophin-linked systems. Each begins with a different first messenger binding to a unique receptor, leading to activation of very different downstream second, third, and subsequent chemical messengers. Having many different signal transduction cascades allows neurons to respond in amazingly diverse biological ways to a whole array of chemical messaging systems. Neurotransmitters (NT) activate both the G-protein-linked system and the ion-channel-linked system on the left, and both of these systems activate genes in the cell nucleus by phosphorylating a protein there called cAMP response element-binding protein (CREB). The G-protein-linked system works through a cascade involving cAMP (cyclic adenosine monophosphate) and protein kinase A, whereas the ion-channel-linked system works through calcium and its ability to activate a different kinase called calcium/calmodulin-dependent protein kinase (CaMK). Certain hormones, such as estrogen and other steroids, can enter the neuron, find their receptors in the cytoplasm, and bind them to form a hormone–nuclear receptor complex. This complex can then enter the cell nucleus to interact with hormone response elements (HRE) there to trigger activation of specific genes. Finally, the neurotrophin system on the far right activates a series of kinase enzymes, with a confusing alphabet soup of names, to trigger gene expression, which may control such functions as synaptogenesis and neuronal survival. Ras is a G protein, Raf is a kinase, and the other elements in this cascade are proteins as well
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


