Overview
Nucleosides and nucleotides are the fourth and final major group of biochemical molecules and are essential for numerous biological functions in humans, including maintaining and transferring genetic information, playing a major role in energy storage, and acting as signaling molecules. These molecules can be divided into two major families—purines, which include adenosine and guanine, and pyrimidines, which include cytosine, thymidine, and uracil. The unique structures and interactions of these molecules serve as the fundamental building block of RNA and DNA molecules and allow fundamental processes of gene replication and protein synthesis to occur. Many other functions of the various nucleosides and nucleotides will be explored in later chapters.
Nucleosides and Nucleotides
Nucleosides and nucleotides are closely involved in the preservation and transmission of the genetic information of all living creatures. In addition, they play roles in biological energy storage and transmission, signaling, regulation of various aspects of metabolism, and even an important role as an antioxidant. Mistakes or deficiencies in their synthesis usually lead to death. Overproduction or decreased elimination of nucleic acid derivates also lead directly to medical conditions.
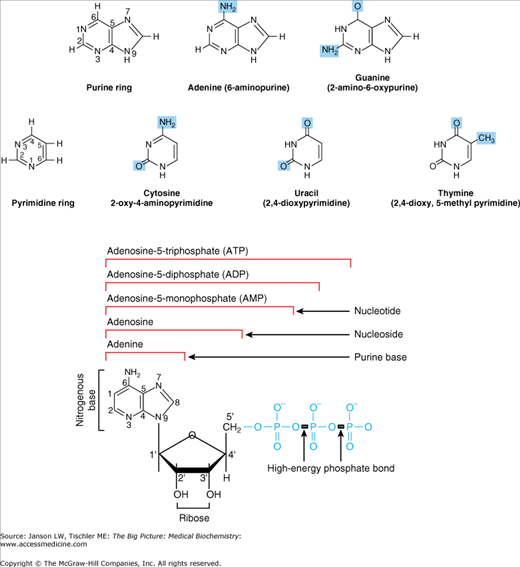
Nucleosides have a nitrogenous base and a five-carbon carbohydrate group, usually a ribose molecule (see Chapter 2). Nucleotides are simply a nucleoside with one or more phosphate groups attached (Figure 4-1). The resulting molecule is found in ribonucleic acid or RNA. If one hydroxyl (OH) group has been removed from the ribose, the deoxy versions of the nucleoside and nucleotide form the building blocks of deoxyribonucleic acid or DNA (Figure 4-1). Each component of nucleosides and nucleotides is discussed below.
Figure 4-1.
Basic Structure of Nucleosides and Nucleotides. Five major nucleoside bases are common in human biology, including the purines (two-ring structure) adenine and guanine (top) and the pyrimidines (one-ring structure) cytosine, uracil, and thymine (middle). Nucleosides (bottom) are made of a nitrogenous base, usually either a purine or pyrimidine, and a five-carbon carbohydrate, usually ribose. A nucleotide is simply a nucleoside with an additional phosphate group or groups (blue); polynucleotides containing the carbohydrate ribose are known as ribonucleotide or RNA. If 2′ hydroxyl group (OH) is removed, the polynucleotide deoxyribonucleic acid (DNA) results. [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
Nitrogenous base—The nitrogenous base of a nucleoside or nucleotide (named because of the nitrogen atoms found in its structure) may be either a purine or a pyrimidine. Purines, including inosine (I), adenine (A), and guanine (G), are two-ring structures and pyrimidines, including uracil (U), cytosine (C), and thymine (T), have only one ring (Figure 4-1). Both purine and pyrimidine nitrogenous bases are made, in part, from amino acids as shown in Figures 4-2 and 4-3, respectively.
Carbohydrate—The carbohydrate component of nucleosides and nucleotides is usually the sugar ribose. When the hydroxyl group is removed from carbon 2 (normally removed after the addition of the nitrogenous base to the carbohydrate), deoxyribose, the sugar molecule found in DNA, results.
Phosphate Group—One or more phosphate groups (PO4−3) may be attached to the carbon 5 of the carbohydrate molecule. The phosphate molecules are important in energy storage and signaling functions of nucleosides and nucleotides.
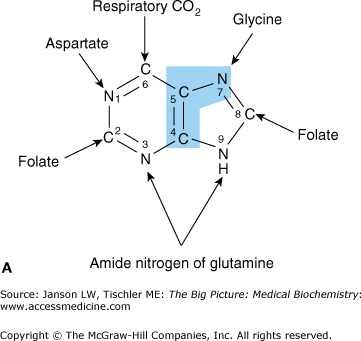
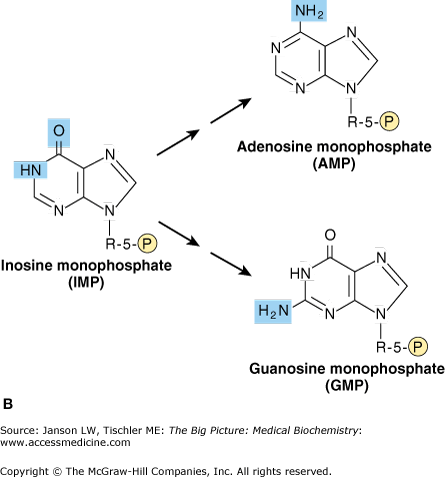
Figure 4-2.
A. Constituents of the Purine Ring. The sources of carbon, nitrogen, and oxygen molecules that form the nitrogenous base of inosine are indicated. Atoms from glycine are indicated in blue shade. B. Synthesis of Adenosine and Guanosine. The nucleosides and nucleotides adenosine and guanosine are produced from the molecule IMP. Synthesis of adenosine requires one additional nitrogen molecule from the amino acid aspartate (blue shade). Guanosine synthesis utilizes one additional nitrogen molecule from the amino acid glutamine (blue shade). [Adapted with permission from Murray RA, et al.: Harper’s Illustrated Biochemistry, 28th edition, McGraw-Hill, 2009.]
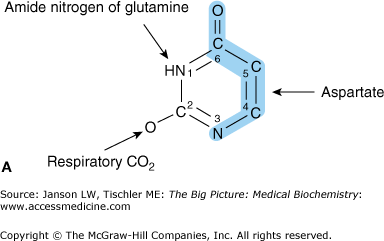
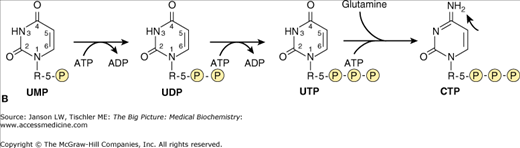
Figure 4-3.
Synthesis of Pyrimidine-Derived Nucleosides Uridine and Cytidine. A. Constituents of the Pyrimidine Uracil Ring. The nucleoside uridine is produced from one aspartate amino acid, one glutamine amino acid, and a bicarbonate molecule from CO2 respiration. Atoms from aspartate are indicated in blue shade. B. Uridine and Cytidine Nucleoside Synthesis. Cytidine synthesis utilizes one additional nitrogen molecule from the amino acid glutamine (indicated by arrow) after the addition of two additional phosphate groups to the UMP molecule. [Adapted with permission from Murray RA, et al.: Harper’s Illustrated Biochemistry, 28th edition, McGraw-Hill, 2009.]
The adenosine- and guanine-based nucleosides and nucleotides are formed by first producing another nucleotide called inosine monophosphate (IMP). IMP is produced from a ribose, parts of one glycine, one aspartate, two glutamine amino acids, two tetrahydrofolate molecules (a modified form of the vitamin folate), and one carbon dioxide (CO2) molecule (Figure 4-2A). After IMP is formed, adenosine monophosphate or guanosine monophosphate may be produced depending on the body’s needs (Figure 4-2B).
The synthesis of purines starts by transference of a phosphate group from an existing adenosine triphosphate (ATP) molecule to a new ribose molecule. As a result of this requirement, the concentrations of ATP must be high for purine synthesis to proceed—a method of regulating when to produce or not produce more of these molecules. Thus, the body only produces more nucleosides and nucleotides when energy levels (from food) are high. Purines can be produced as above, or can be obtained from the diet or by breaking down and recycling existing nucleosides and nucleotides. Mammals, including humans, preferentially use the recycling pathway when appropriate starting materials are available.
Unlike purines, the pyrimidine nitrogenous bases, uracil and cytosine, are formed before bonding to the carbohydrate portion of the nucleotide. The process starts by producing the uracil ring (Figure 4-3A) in a three-step process utilizing one each of the amino acids glutamine and aspartate and one bicarbonate molecule (HCO3−). Next, cytidine triphosphate (CTP) is derived from the nucleotide uridine monophosphate (UMP) after the conversion of UMP to the triphosphate form (uridine triphosphate); an extra nitrogen is gained from one additional glutamine amino acid (Figure 4-3B, far right). Cytidine monophosphate (CMP) and diphosphate (CDP) can be produced from (CTP) by the subsequent loss of two or one phosphate groups, respectively. The final pyrimidine-derived nucleotide, thymidine, found only in DNA, is produced by a separate process involving the deoxy form of UMP (dUMP). This process will be discussed below.