Overview
The digestive or gastrointestinal system is roughly defined as the anatomical component from the mouth to the anus, including organs responsible for transit, mechanical breakdown, digestion and absorption of foodstuffs, as well as the efficient elimination of solid waste. Included are the mouth and dentitia, pharynx and esophagus, stomach, small intestine, liver, gall bladder, pancreas, large intestine, rectum, and anus. As with the complex integration and control of metabolism, the digestive system is, itself, under the influence of neurological and hormonal regulation that both activates and inhibits many of its complex actions. Most of these complex actions are, themselves, simple biochemical processes of proteins, carbohydrates, lipids, and nucleosides/nucleotides to include enzyme reactions with associated activating and inhibitory molecules, membrane-spanning protein channels, and pumps all leading to the production and storage of energy and essential building blocks for current or future use.
Summary of the Digestive System
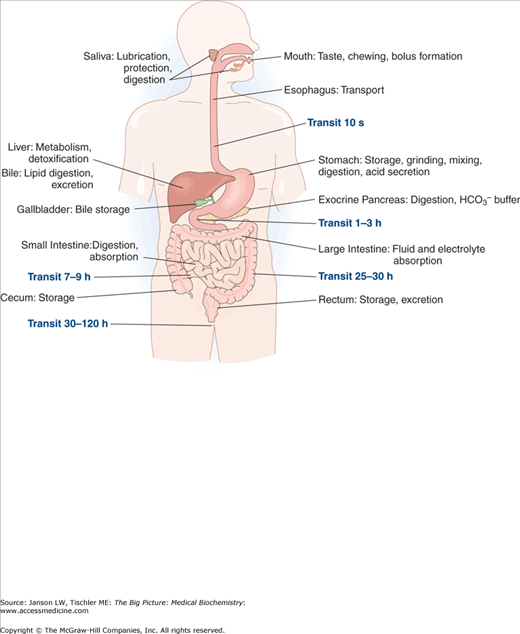
The digestive system is the collection of organs responsible for the digestion of ingested foods and liquids (Figure 11-1). This system is classically considered to start at the mouth and continue via the esophagus, stomach, small and large intestines, and end at the rectum/anus. Besides their mechanical and enzymatic breakdown of foodstuffs, considerable contributions toward digestions are supplied from the liver and pancreas.
Figure 11-1.
Overview of the Digestive System. Components of digestion and transport of food from the mouth to the rectum/anus are shown, including a summary of their contributions and the average amount of time for food to reach their location after ingestion. [Reproduced with permission from Kibble JD and Halsey CR: The Big Picture: Medical Physiology, 1st edition, McGraw-Hill, 2009.]
Mouth
Teeth provide the initial mechanical breakdown of food via chewing or mastication and, therefore, the formation and health of teeth is important. Proteins serve as an important structural component of teeth enamel. Like bone, teeth require the collagen-like proteins amelogenin (over 90% composed of proline, glutamine, and histidine amino acids), ameloblastin, enamelin, and tuftelin (a phosphorylated glycoprotein), which organize, initiate, and direct calcium phosphate crystal formation and help anchor teeth to the gums.
The possible breakdown of enamel is equally important and is also dependent on biochemical processes. Dental plaque formation and tooth decay both rely on enzymatic processes for formation as well as prevention. Plaque is caused mainly by the normal oral bacteria Streptococcus mutans, Lactobacillus acidophilus, Fusobacterium nucleatum, Actinomyces viscosus, and Nocardiaspp. When these organisms form a layer on teeth, those closest to the teeth exist in an oxygen-deficient environment and convert to anaerobic respiration for energy production. This enzymatic process turns carbohydrates into lactic acid from pyruvate (Chapter 6) and results in a pH of below 5.5, leading to tooth decay, the demineralization process that causes cavities. Glucose, fructose, and especially sucrose (common table sugar) are the main carbohydrate sources.
The mouth is also the location for the start of the processes of carbohydrate, lipid, and protein digestion via important enzymes contained in saliva. Saliva production, along with feelings of hunger and satiety, is controlled by a variety of neurobiochemical processes, which start with the initial thoughts or cephalic phase of eating. The physical presence and act of chewing and tasting food, known as the orosensory or gustatory phase, elicits further signals that enhance saliva formation and expression. The various factors affecting hunger and satiety are discussed in Chapter 19. Saliva also traps molecules produced by normal oral bacteria that adds to the taste sensation of otherwise odorless and tasteless food compounds. The hormone gustin, produced in saliva and an activator of a calmodulin-dependent cyclic adenosine monophosphate (cAMP) phosphodiesterase (Chapter 8), is also thought to play an important part in taste bud formation. Digestive enzymes contained in saliva and their roles in digestion are listed in Table 11-1.
Item | Function/Notes |
|---|---|
Water | 98%–99% of total |
Electrolytes | 1%–2% of total: sodium (2–21 mM), potassium (35 mM), calcium (1.2–2.8 mM), magnesium (0.08–0.5 mM), chloride (5–40 mM), bicarbonate (25 mM), and phosphate (1.4–39 mM) |
Major Digestive Enzymes | |
α-amylase | Initiates random digestion of amylose and amylopectin chains, producing maltotriose, maltose, amylose, glucose, and oligosaccharides. |
Lingual lipase (secreted by tongue) | Initiates hydrolysis of long-chain triglycerides into diacylglycerol and free fatty acids, which continues into and through stomach. Optimal activation requires acidic (∼pH 4) environment, so vast majority of activity is in stomach. |
Minor Digestive Enzymes | |
Salivary acid phosphatases A + B | Removes phosphate group from proteins by reaction orthophosphoric monoester + H2O → alcohol + H3PO4. |
N-acetylmuramoyl-l-alanine amidase | Removes N-acetylmuramoyl from amino acid residue in certain glycoproteins. |
NAD(P)H dehydrogenase (quinone) | Catalyzes the following reaction: NAD(P)H + H+ + a quinone ⇌ NAD(P)+ + a hydroquinone |
Superoxide dismutase | Changes highly reactive superoxide molecules into oxygen and hydrogen peroxide (H2O2) by oxidation of a metal ion (e.g., copper, zinc, manganese, iron, or nickel). |
Glutathione transferase | Detoxifies several types of molecules containing the sulfur-containing, tripeptide glutathione, produced from the cysteine, glutamic acid, and glycine amino acids, via reduction and conjugation. |
Aldehyde dehydrogenase (class 3) | Catalyzes the oxidation (dehydrogenation) reaction RCHO + NAD+ + H2O → RCOOH + NADH + H+ |
Glucose-6-phosphate isomerase | Changes glucose-6-phosphate into fructose 6-phosphate. |
Antibacterial Compounds | |
H2O2 | Highly reactive oxygen-containing molecule. |
Immunoglobulin A | Main immunoglobulin in saliva; plays a critical role in mucosal immunity. |
Antimicrobial Enzymes | |
Lactoferrin | Produces small peptides, called lactoferricin and kaliocin-1, which, coupled with iron, inhibit bacterial and viral binding to cell membranes. Also exhibits antifungal properties. |
Lactoperoxidase (salivary) | Kills bacteria via formation of reactive bromine and iodine species. |
Lysozyme | Hydrolyzes peptidoglycan component of bacterial cell wall at 1,4-β-linkages between N-acetylmuramic acid and N-acetyl-d-glucosamine residues. |
Thiocyanate (SCN)− | Reactive sulfur-containing molecule |
Mucus | Secretion offering lubrication and protection to teeth, tongue, and epithelial cells of the gums/inner mouth as well as the remainder of the digestive tract. |
Mucopolysaccharides | Long and unbranched polysaccharides consisting of a repeating disaccharide unit, often referred to as Glycosaminoglycans. See Chapter 2. |
Glycoproteins | Proteins with large, attached carbohydrate chains. See Chapter 2. |
Cells | ∼8 million human/ml; ∼500 million bacterial/ml. Bacterial metabolism leads to production of thiols, amines, and organic acids causing bad breath (halitosis). |
“Meth Mouth”: Patients who abuse methamphetamines are prone to marked dental decay, known colloquially as “Meth Mouth.” Methamphetamine acts on the α-adrenergic receptors of the vasculature of the salivary glands, causing vasoconstriction and reducing salivary flow, depriving the oral environment of saliva’s buffering activity to counteract acidity and prevent demineralization of enamel. Methamphetamine-induced vomiting also exposes teeth to acids. In addition, methamphetamine overstimulates the sympathetic nervous system, eventually depleting norepinephrine and dopamine and altering concentrations of other central nervous system (CNS) neurotransmitters such as serotonin, acetylcholine, and glutamate. This reduction increases the demand for adenosine triphosphate (ATP); methamphetamine users may compensate by consuming more carbohydrates in the form of sugars and starches. Observers specifically report a high intake of carbonated soft drinks among meth users. At the same time, users typically abandon oral hygiene. In short, methamphetamine use encourages an environment that maximizes caries risk—decreased saliva, frequent exposure to sugar, and lack of plaque control. |
Stomach
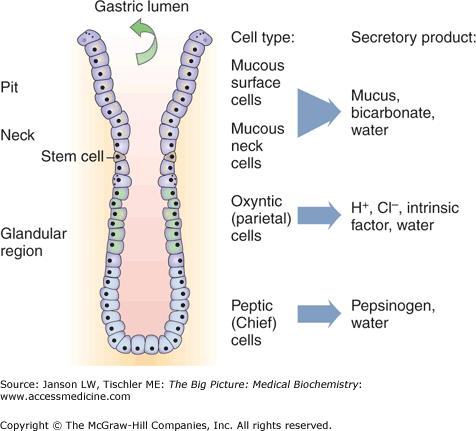
Stomach is the location of continued mechanical breakdown of food via the actions of its smooth muscle layers but, perhaps more importantly, the site of the activation and activity of a number of regulated enzymes and exposure to acid (pH 1–2) that initiates metabolism. The other main function of the stomach is the regulated and coordinated secretion of hydrogen atoms and various digestive enzymes under the control of the autonomic nervous system and several hormones. These molecules are produced from a variety of cell types found in various parts of the stomach as shown in Figure 11-2 and summarized below. Hormones that affect the stomach are described in Table 11-2.
Mucus (Neck) Cells. Found in all parts of the stomach, these cells produce a viscous mixture of protective enzymes and mucins, large glycoproteins (Chapter 1), via stimulation of a myristylated alanine-rich C kinase. Mucins have low glycosylation on the amino and carboxy terminal ends but very high levels of glycosylation (via serine, threonine, and asparagine amino acids) in the central part of its amino acid sequence. These mucin glycoproteins are also interlinked by cysteine–cysteine disulfide bonds (Chapter 1) that form large aggregate gels filled with water and protected from enzymes by their dense carbohydrate coating. The secreted mucus provides a protective barrier against the digestive enzymes and highly acidic conditions found in the stomach.
Parietal (Oxyntic) Cells. Found in all parts of the stomach, parietal cells are stimulated by the hormones histamine via the H2 receptor (Gs—adenyl cyclase/cAMP) and gastrin via a CCK2 receptor [Gq—phospholipase C/inositol triphosphate (IP3)/Ca2+] as well as by the vagus (parasympathetic) nerve via acetylcholine and the M3 receptor (Gq—phospholipase C/IP3/Ca2+). Stimulation of adenyl cyclase is known to specifically activate the H+/K+ ATPase active transport channel and gastric acid production. These cells produce three main components that are as follows:
- a. Gastric acid [mainly hydrogen (H+) and chloride (Cl−) ions] via a unique H+/K+ ATPase active transport channel that pumps hydrogen ions into the stomach against a very high concentration gradient (approx. 3 million to 1). Gastric acid functions in protein denaturization, pepsinogen activation (see below), and inhibition of bacterial growth.
- b. Bicarbonate ion (HCO3−). Excreted into the blood as part of the overall H+ pumping mechanism.
- c. Intrinsic factor. Required for intestinal absorption of vitamin B12 (see Chapter 10).
Chief (Zymogenic) Cells. Produce pepsinogen, the proenzyme form of the important enzyme pepsin, which cleaves peptide bonds, preferably at hydrophobic and aromatic [phenylalanine (Phe), tryptophan, and tyrosine] amino acids. Stimulation of chief cell secretions is by the vagus nerve, acidic conditions per gastric acid, or by the hormones gastrin or secretin (produced in the duodenum). In infancy, Chief cells also produce the enzyme rennin, which aids milk absorption by breaking the Phe–methionine peptide bond in milk protein kappa-casein. Secretion of rennin is stimulated by ingestion of milk by the human infant but the gene product is turned off past this stage.
Enterochromaffin-like Cells (ELCs). Found in the gastric glands. These cells secreted histamine, which activates parietal cells and gastric acid production. ELCs are, themselves, activated by the hormone gastrin, pituitary adenyl cyclase-activating peptide, and vagus nerve. ELCs are inhibited by somatostatin.
G Cells. Found in the antrum. Secrete the hormone gastrin (see Table 11-2), which both increases the secretion of and works along with histamine to stimulate parietal cells to produce hydrochloric acid and Chief cells to produce pepsin. Secretion of gastrin is increased by parasympathetic vagus nerve activity via release of gastrin-releasing peptide or by the presence of amino acids in the stomach.
Prostaglandin E2. Binding to its receptor stimulates smooth muscle contraction of the gastrointestinal tract and decreases parietal cell secretion of gastric acid while increasing mucus production. The action is via the Gi protein receptor, which inhibits the production of cAMP by adenyl cyclase and, therefore, parietal cell H+/K+ ATPase pump activity.
Figure 11-2.
Hormone Production from the Stomach. Specific portions of the stomach are responsible for production of hormones as illustrated above (see text for further description). [Reproduced with permission from Kibble JD and Halsey CR: The Big Picture: Medical Physiology, 1st edition, McGraw-Hill, 2009.]
Molecule | Location Produced | Action |
|---|---|---|
Cholecystokinin (CCK) | Peptide hormone produced by cells in the duodenum | Main effect is the contraction of smooth muscle of the gall bladder and simultaneous secretion of pancreatic enzymes to increase digestion. However, emptying of the stomach and gastric acid secretion is also decreased by CCK as digestion progresses beyond the stomach. |
Enteroglucagon | Mainly in terminal ileum and colon from the prohormone preproglucagon | Decreases the production of gastric acid by parietal cells and smooth muscle contraction (motility) of the stomach, thereby decreasing gastric emptying. |
Gastrin | Produced by G-cells in response to presence of undigested proteins and/or distension of the antrum of the stomach | Inhibited by pH <4 and by the hormone somatostatin (see below). Gastrin stimulates hydrochloric acid, pepsinogen, and intrinsic factor secretion from parietal cells, pepsinogen/rennin by Chief cells, as well as histamine release from enterochromaffin-like cells. Smooth muscle contraction (i.e., motility) of the stomach is also enhanced by gastrin. |
Gastric inhibitory peptide (GIP) | Peptide hormone produced in the duodenum/jejunum | Decreases gastric acid release by parietal cells as well as smooth muscle contraction (motility) of the stomach. GIP may also increase insulin secretion and fatty acid metabolism (e.g., milk digestion) by activating lipoprotein lipase. |
Motilin | Peptide hormone made mainly in the duodenum/jejunum | Increases smooth muscle contraction (fundus, antrum, and gall bladder). Also, stimulates secretion of somatostatin, pancreatic peptide, and pepsinogen. |
Secretin | Produced in the duodenum | Several effects including increased secretion of water and bicarbonate as well as insulin (following glucose intake) from the pancreas and bile from the liver. In the stomach, secretin inhibits G-cell production of gastrin, thereby reducing the acidity (pH) of digested food leaving the stomach and entering the duodenum. Lowered pH allows maximal activation of pancreatic enzymes being secreted into this part of the small intestine. Secretin also enhances secretion of pepsin as well as the hormones glucagon, pancreatic polypeptide, and somatostatin. |
Somatostatin/growth inhibiting hormone | Produced in the stomach, intestines, and pancreas | Decreases release of gastrin, CCK, secretin, motilin, vasoactive intestinal peptide (VIP), GIP, and enteroglucagon, decreasing stomach secretion and contraction. |
VIP | Peptide hormone produced in digestive tract and brain | Causes relaxation of smooth muscle (lower esophageal sphincter, stomach, and gallbladder), stimulates pepsinogen secretion, dilutes bile and pancreatic juice, increases bicarbonate production in the pancreas, decreases gastrin-induced gastric acid secretion, and increases water secretion in intestine. |
Achlorydia: The destruction or damage of parietal cells leads to a marked reduction in the production of gastric acid, essential for the initial breakdown of food and activation of stomach enzymes (e.g., pepsin). Although several disease states and/or surgical interventions can affect parietal cells, immune destruction of the cells leads to the condition of achlorydia/hypochloridia in which decreased amounts of hydrochloric acid are produced. The resulting higher stomach pH leads to symptoms of gastroesophageal reflux, pain and fullness from inadequate digestion, and increased growth of bacteria, normally limited by low pH, which can lead to diarrhea and decreased absorption of essential ions (e.g., magnesium and zinc) and vitamins (e.g., C, K, B-complex), which themselves lead to other disease states. Treatment is via supplementation with Betaine HCl, a form of hydrochloric acid, which survives into the stomach, and any required vitamins and minerals. |
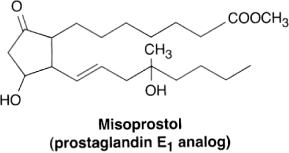
Misoprostol and Gastric Ulcers. Misoprostol (see the figure), a synthetically produced prostaglandin E1, is sometimes used to prevent gastric ulcers because of its ability to inhibit parietal cell production of gastric acid. Misoprostol is normally used only for treatment of or prophylaxis against nonsteroidal anti-inflammatory drug-induced peptic ulcers because other medication classes (H2-receptor blocker and protein pump inhibitors, PPIs) are more effective for long-term care of acid reflux and similar disorders.
Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009. |
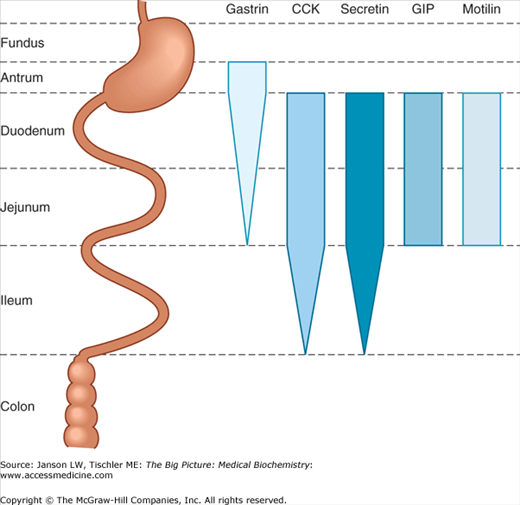
The actions of each of the above cell types and, therefore, the environment of the stomach are controlled by vagus nerve signals as well as the hormones listed below. Of note is the fact that several hormones that affect the stomach are produced and also act on organs outside the stomach (e.g., small intestine) to decrease stomach motility and/or digestion (Figure 11-3). In this manner, the body not only “turns on” the stomach when food is present but also turns it “off” when food has traveled further along the digestive tract.
Figure 11-3.
Hormone Production by the Digestive System. Sites of production of the five major gastrointestinal hormones along the length of the gastrointestinal tract. The width of the bars indicates the relative abundance at each location. CCK, cholecystokinin; GIP, gastric inhibitory peptide. [Reproduced with permission from Barrett KE, et al.: Ganong’s Review of Medical Physiology, 23rd edition, McGraw-Hill, 2010.]
Get Clinical Tree app for offline access