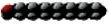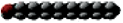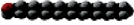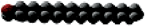Overview
Lipids are the third major type of biochemical molecule found in humans. Although one of their major functions relates to the formation of biological membranes (phospholipids and cholesterol), lipid molecules are also essential for energy storage and transport (triacylglycerols), cellular binding and recognition and other biological processes (glycolipids), signaling (steroid hormones), digestion (bile salts), and metabolism (fatty acids, ketone bodies, and vitamin D). Lipid molecules are mainly hydrophobic and are, therefore, found in areas away from water molecules or are involved in mechanisms such as lipoprotein complexes that allow their movement in and through water environments. The smaller hydrophilic parts of lipids are, themselves, important in formation of biological membranes and in the several specific functions of lipids and lipid-derived molecules.
Basic Lipid Functions
A major role of lipid molecules is to provide the building blocks for biological membranes, including phospholipids, glycolipids, and cholesterol. However, other lipids known as triacylglycerols (also referred to as triglycerides or fats) function in the storage of biological energy and bile salts, derived in the liver from cholesterol and serve in the digestion of dietary fat. Finally, several lipid-derived molecules serve as important hormones and intracellular messengers.
It is important to note that the major part of every lipid molecule is hydrophobic in nature and, like the hydrophobic parts of proteins discussed earlier (Chapter 1), prefers to be away from and protected against interaction with water molecules. This hydrophobic character is fundamental in membrane formation, lipid transport, and in many of the functions that the various types of lipid molecules perform.
Basic Membrane Lipid Structure
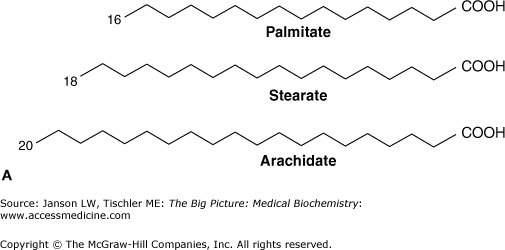
Fatty acids are composed of long chains of carbon molecules with a carboxylic acid (COOH) at carbon 1 and a CH3 (methyl) group at the end of the chain (Figure 3-1A). The carboxylic acid group is involved in bonding of the fatty acid to the other components of a lipid molecule. In humans, fatty acids are usually 12–24 carbons long and most often the number of carbons in the fatty acid backbone is even. Fatty acids can contain single (C—C), double (C≕C), or triple (C≡C) carbon–carbon bonds.
- Saturated fatty acids contain only single carbon–carbon bonds, and all of the carbon molecules are bonded to the maximum number of hydrogen molecules.
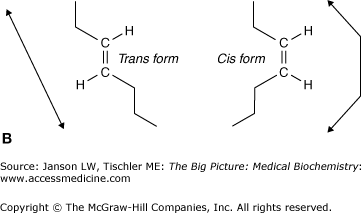
- Unsaturated fatty acids have at least one double carbon–carbon bond with the potential for additional hydrogen atom bonding still existing for some of the carbon atoms in the backbone chain. If more than one double bond is present, the term polyunsaturated is used. These double bonds can exist in either a “kinked” cis double bond or a more linear trans double bond (Figure 3-1B-C).
- Saturated fatty acids contain only single carbon–carbon bonds, and all of the carbon molecules are bonded to the maximum number of hydrogen molecules.
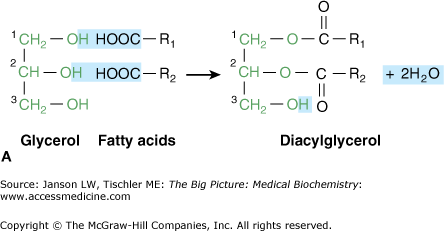
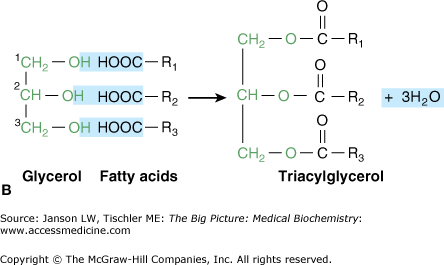
Glycerol is a simple three-carbon molecule with hydroxyl groups at each carbon (Figure 3-2A). These hydroxyl groups are the reactive location where fatty acids and other components of a lipid molecule bond to form diacylglycerol (Figure 3-2A) and triacylglycerol (Figure 3-2B) molecules.
| Print
Double Bonds and Melting Temperatures: The number and type of double bonds in a particular lipid molecule’s fatty acid affects how that fatty acid “packs” with other fatty acids and, therefore, the temperature at which the particular lipid molecule melts. For example, the saturated fatty acids listed in Table 3-1 have melting points between 44°C and 77°C. The unsaturated fatty acids have far lower melting points, ranging from 13°C to −50°C, decreasing as the number of double bonds (polyunsaturation) increases. “Kinked” cis double bonds make the packing of fatty acids even more disorganized and lower the melting temperature even further. The effects of saturated/unsaturated/polyunsaturated and cis/trans fatty acids are readily seen in the different melting temperatures of butter, composed of a highly saturated lipids, and margarine, composed of unsaturated lipids. The linear nature of trans fatty acids makes them similar to the structure of saturated fatty acids. This structural feature, relative to the cis configuration, seems to make metabolism of trans fats difficult. Consequently, trans fats remain longer in the circulation, thereby contributing to arterial deposition and subsequent development of coronary heart disease. In general, unsaturated fats are healthier for the human body and, therefore, dietary fats composed mostly of cis double bonded, polyunsaturated fats are recommended by dieticians and clinicians to help avoid heart disease and other medical problems.
| Print
Essential Fatty Acids: Much like there are essential amino acids that the body can only get from dietary sources, certain fatty acids are also deemed essential. Two particular essential fatty acids, linoleate and linolenate, have double bonds at the sixth and third carbon atoms counting from the methyl end of their chains, respectively, and are needed to produce certain 20-carbon long fatty acids containing double bonds. These two fatty acids are known as omega-6 (ω-6) and omega-3 (ω-3) fatty acids. Humans do not have the ability to produce double bonds at these locations and, therefore, must obtain these two required fatty acid building blocks from vegetable oils. Arachidonate with a 20-carbon chain and four cis double bonds is also an essential fatty acid involved in several important biological functions. Recently, longer carbon chain ω-3 fatty acids have been proposed to decrease heart attacks and strokes; supplements and some food products are now available, which contain these fatty acids. Interestingly, excessive dietary intake of ω-6 fatty acids has been implicated in an increased risk of heart attacks, strokes, some cancers, and even depression.
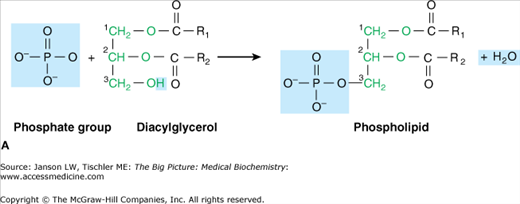
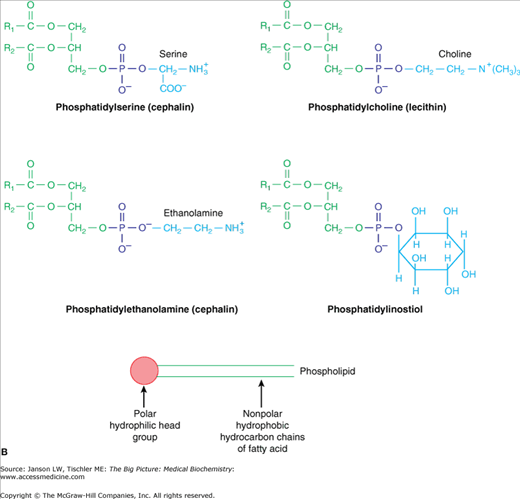
Head Group The final component of a lipid molecule varies with each type of lipid and, along with the two specific fatty acids, defines each particular lipid. This third part of the lipid molecule is often called the “head group,” aptly named if one envisions the end methyl group of the fatty acid chains to be the tail of the lipid molecule (Figure 3-3A). Most lipids in a biological membrane have a phosphate group (PO4−3) attached to the third glycerol carbon and are, therefore, called phospholipids. Usually, an additional molecule (several common examples found in humans and the resulting phospholipid molecules are shown in Figure 3-3B) is attached to the phosphate molecule, resulting in the final head group of the lipid molecule. This head group is usually charged, creating a part of the lipid that is hydrophilic, and wants to be near water, a quality that is essential for the formation of biological membranes (Chapter 8) and many lipid functions.
Figure 3-1.
A. Common Saturated and Unsaturated Fatty Acids. Palmitate (16-carbon), stearate (18-carbon), and arachidate (20-carbon), fatty acids, shown by the carbon backbone and in “stick diagram” form for arachidate often used for simplicity. The carbon atoms of fatty acids are numbered from the carboxylic acid (COOH) to the terminal methyl (CH3) group. Hydrogen atoms are not shown for clarity. B. Fatty Acid Chain Double Bonding. Detail of unsaturated fatty acid carbon chain, illustrating trans double bond (left: hydrogen atoms on opposite sides of the bond and resulting linear carbon chain) and unsaturated cis double bond (right: hydrogen atoms on the same side of the bond and resulting “kinked” carbon chain). C. Common Unsaturated Fatty Acids. (Top and middle) Two 18-carbon unsaturated acids showing a cis double bond (oleic acid) and a trans double bond (elaidic acid) both at carbon 9. Arrows illustrate different conformations of fatty acid chain that result from the two types of saturated bonds. (Bottom) An 18-carbon unsaturated fatty acids with two cis double bonds (linoleic acid) at carbons 9 and 12 (see arrows). Note how the addition of a second cis double bond creates an even more nonlinear fatty acid chain, which causes increased disorder of packing and, therefore, increased fluidity of biological membranes. [Adapted with permission from Murray RA, et al.: Harper’s Illustrated Biochemistry, 28th edition, McGraw-Hill, 2009.]
Figure 3-2.
A–B. A. Glycerol, Diacylglycerol, and Triacylglycerol. Glycerol is a simple, three-carbon chain molecule (green) with a hydroxyl group (OH) bonded to each of the carbon atoms. The hydroxyl groups at carbons 1 and 2 of glycerol bond react with the carboxylic acid groups (COO−) of the fatty acid chains, resulting in two new bonds and two water (H2O) molecules. In general, unsaturated fatty acids bond to carbon 1, whereas saturated fatty acids bond to carbon 2. The resulting molecule is called “diacylglycerol” and is involved in important signaling pathways (Chapter 8). B. Triacylglycerol is formed when a third fatty acid bonds to the third glycerol hydroxyl group. This resultant molecule is an important storage form of energy (Chapter 8). [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
Figure 3-3.
A. Phospholipid Components and Formation. Bonding between hydroxyl (OH) of a phosphate group with the hydroxyl (OH) of glycerol carbon 3 (green) results in a phospholipid molecule and one water molecule. The basic phospholipid illustrated above is termed phosphatidic acid and is the building block of common phospholipids found in the cell membrane (see below). Fatty acid chains are depicted in black. B. Common Phospholipids Found in Humans. Common head groups, which form phospholipids (top and middle) and are shown in blue, bonded to the hydroxyl (OH) of a phosphate group (purple), which itself is bonded to the hydroxyl (OH) of glycerol carbon 3 (green). This structure results in a phospholipid molecule that is generally found in membranes. Lipid molecules are often represented in “stick diagram” form (bottom) with the charged phosphate group and hydrophilic head group shown as a circle and oval and with the fatty acid “tails,” depicted as either straight or jagged lines, forming the hydrophobic region. [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
Name | Carbon Atoms | Shape | Name | Carbon Atoms | Double Bonds | Shape | ||
|---|---|---|---|---|---|---|---|---|
Saturated | Laurate | 12 |
| Unsaturated | Palmitoleate | 16 | 1 (cis) at carbon 9 |
|
Myristate | 14 |
| Oleate | 18 | 1 (cis) at carbon 9 |
| ||
Palmitate | 16 |
| Linoleate | 18 | 2 (cis) at carbons 9 and 12 |
| ||
Stearate | 18 |
| Linolenate | 18 | 3 (cis) at carbons 9, 12, and 15 |
| ||
Arachidate | 20 |
| Arachidonate | 20 | 4 (cis) at carbons 5, 8, 11, and 14 |
| ||
Behenate | 22 |
| ||||||
Lignocerate | 24 |
|
Complex Lipids
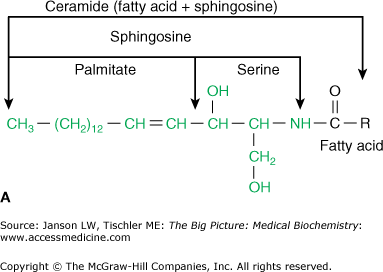
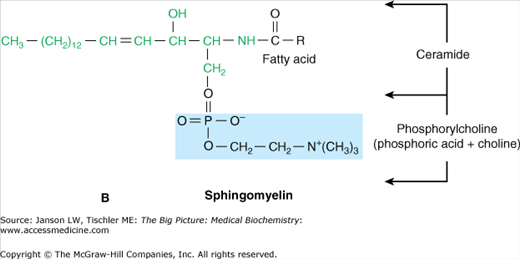
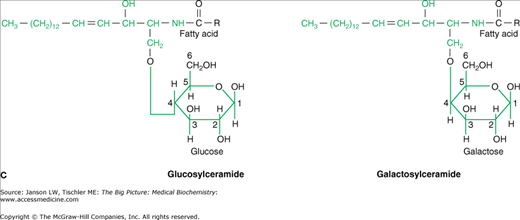
Just as carbohydrate and protein molecules can bind together (discussed in Chapter 2), carbohydrates can also bind to lipids to form a glycolipid. However, in a human glycolipid, the glycerol backbone is generally replaced by a backbone of sphingosine [made from the amino acid serine and the 16-carbon fatty acid palmitate (Figure 3-4A)] and is, therefore, referred to as a sphingolipid. Sphingosine can bind two other molecules with the remaining hydroxyl (OH) and amino (NH3) groups from the serine amino acid. In human sphingolipids, the amino group is always bound to another fatty acid to make the molecule ceramide. From ceramide, the particular molecule(s) attached to the remaining hydroxyl group defines both the name and the characteristics of the resulting sphingolipid. For example, the molecule sphingomyelin, which can make up to 20% of the total phospholipid in many biological membranes, is made of ceramide and a phosphoryl choline head group (Figure 3-4B).