Overview
Connective tissue provides a framework and support for a large variety of structures, including organs, blood vessel walls, as well as the better known functions of connecting muscle to bone and bone to bone. Chondrocytes are responsible for the production of connective tissue components, including the principal protein, collagen. The three types of connective tissues offer a wide variety of functions to fulfill the many roles needed by the body. A multitude of diseases result from abnormalities, deficiencies, or overproduction of connective tissues or their components.
Bones provide a mechanical structure for the human body and, in that role, also allow effective muscle contraction and, therefore, movement. Bones offer protection for the body’s internal organs, especially brain, heart, lungs, liver, stomach, and spleen. In the ear, bones transduct sound waves from the ear drum to the inner ear. Osteoblasts, osteocytes, and osteoclasts produce, break down, remodel, and repair both the organic and inorganic matrix that make up bones. In doing so, these cells, as well as several regulatory molecules, help to regulate calcium and phosphate metabolisms and levels in the body. Bones also have a synthetic function. Within their marrow, bones produce red and white blood cells as well as growth factors; and store fatty acids as yellow marrow. Finally, bones provide for the storage of certain minerals, including calcium and phosphorus and, to a lesser extent, zinc, copper, and sodium. In an analogous role, bone can temporarily absorb and store toxic heavy metals to reduce their effects on the body.
Connective Tissue
Connective tissue is a fibrous tissue made mainly of collagen (Chapter 1) and proteoglycans (Chapter 2) that forms, supports, and/or connects various organs in the body, attaches muscles to bones (e.g., tendons) and bones to bones (e.g., ligaments), forms the supportive matrix during bone formation (see below), and makes up various structures such as parts of blood vessels and intestinal walls. One major example of connective tissue is collagen, which is found in various forms throughout the body (Figure 13-1A–D).
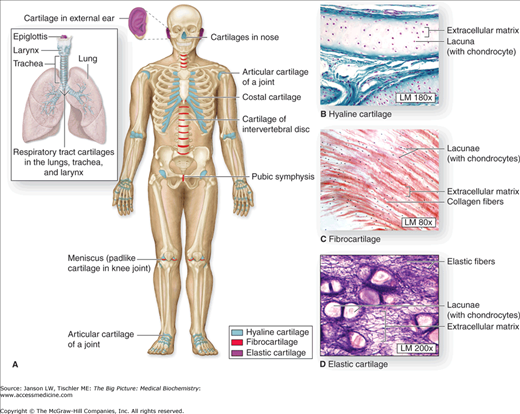
Figure 13-1.
A–D. Distribution of Cartilage in Adults. (A) There are three types of adult cartilage distributed in many areas of the skeleton, particularly in joints and where pliable support is useful, as in the ribs, ears, and nose. Cartilage support of other tissues throughout the respiratory system is also prominent. The photomicrographs show the main features of (B) hyaline cartilage, (C) fibrocartilage, and (D) elastic cartilage. [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
Formation of connective tissue relies on chondrocytes, which both produce and maintain the collagen matrix. Chondrocytes differentiate from osteochondrogenic cells, which can alternatively develop into osteoblasts (see below). Although the different structures vary widely, three major fiber forms of connective tissue fibers are prominent—collagenous fibers, elastic fibers, and reticular fibers.
Collagen is the principal component of collagenous fibers, and the type of collagen determines the structural and functional qualities of that particular form. Collagen is believed to represent about a quarter of the total protein in human body. Although 29 types of collagen have been discovered, each varying in its structure, location in parts of the body, and functions, four (types I–IV) make up a vast majority of connective tissue. Structure and formation of a type I collagen fiber is illustrated in Figure 13-2A–B.
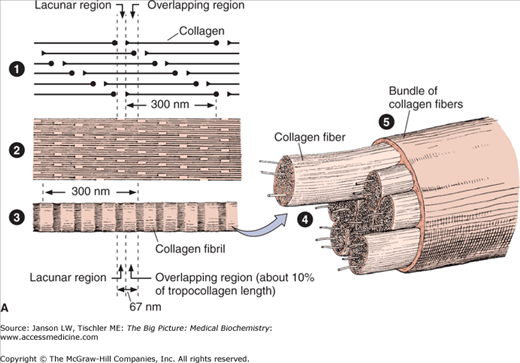
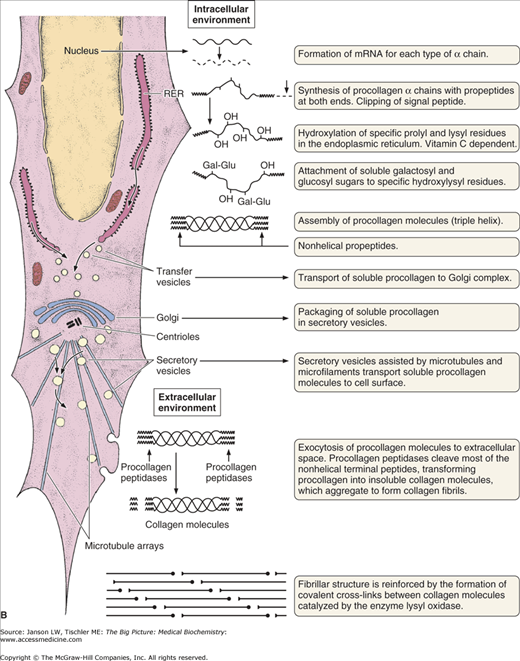
Figure 13-2.
A. Structure of Collagen. Illustration of structural arrangement of individual, overlapping collagen molecules (1), collagen fibrils, collagen fibers (2 and 3), and collagen fiber bundles (4 and 5). The overlapping nature of this structural organization produces the required strength and flexibility of this molecule. [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.] B. Formation of Collagen Fibers. Collagen peptides are synthesized by ribosomes in the lumen of rough endoplasmic reticulum (RER) as “preprocollagen” molecules (not shown) with N-terminal signal peptides. Signal peptides are removed to produce “procollagen” molecules. Proline and lysine amino acid residues are hydroxylated via prolyl hydroxylase and lysyl hydroxylase enzymes, which depend on vitamin C as a cofactor. Some amino acid residues are also glycosylated. The procollagen molecules form a left-handed triple helix within the RER lumen. The helical molecules are transported to the Golgi apparatus, where they are processed and secreted via exocytosis to the cell exterior. The amino- and carboxy-terminal ends are removed by the enzyme procollagen peptidase to form collagen fibrils with further cross-linking of hydroxylysine and lysine residues on different tropocollagen molecules by the enzyme lysyl oxidase. Cross-linked collagen fibrils form collagen fibers. Because there are many slightly different genes for procollagen α chains and collagen production depends on several posttranslational events involving several other enzymes, many diseases involving defective collagen synthesis have been described. [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
Varying combinations of the different types of collagen can alter this basic structure for particular cell functions. Most collagenous fibers follow the previously described, left-handed, triple-helix of type I (Figure 13-3A), with multiple glycine, proline/hydroxyproline, and hydroxylysine amino acids contributing to its structure.
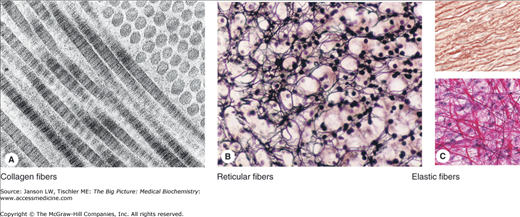
Figure 13-3.
A–C. Representative Types of Collagen-Based Connective Tissue. (A) Molecules of type I collagen, the most abundant type, assemble to form much larger structures. Transmission electron microscopy shows that fibrils cut longitudinally and transversely. In longitudinal sections, the fibrils display alternating dark and light bands, which are further divided by cross-striations, and in cross section, the cut ends of individual collagen molecules can be seen. (B) Silver-stained sections of adrenal cortex illustrate a network of reticular fibers, which provides a framework for cell attachment. Reticular fibers contain type III collagen, which is heavily glycosylated. (C) Elastic fibers are composed of a third type of connective tissue, formed from microfibrils of elastin and fibrillin and add resiliency to the connective tissue. They can be seen between layers of smooth muscles in the wall of elastic arteries such as the aorta (pink, upper panel) and in structures such as mesentery (dark magenta, lower panel). [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
The second type of connective tissue is the reticular fiber, composed of type III collagen and forming an ordered, “reticulum” meshwork instead of a linear structure (Figure 13-3B). The reticular network links these proteins to carbohydrates, including glucose, galactose, mannose, and fucose (Chapter 3). Reticular fibers are found mainly in liver, muscle, bone marrow, lymph system, and various other tissues and organs, where it offers a supportive framework.
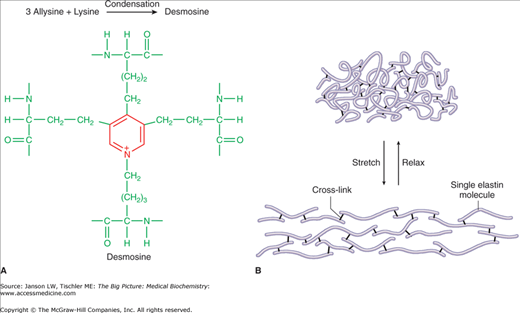
The final type of connective tissue fiber is the elastic fiber (Figure 13-3C). Elastic fibers are formed from smaller microfibrils, made mainly of the protein tropoelastin/elastin and the glycoprotein fibrillin, and cross-linking polypeptides (Figure 13-4A–B). The microfibrils are in the form of irregular, random coils, with glycine, valine, alanine, proline, and lysine amino acids contributing to the stable structure.
Figure 13-4.
A–B. Quaternary Structure of Elastic Fibers. Elastin microfibrils may form internal and external (between different elastin fibers) cross-links to form the cross-link structure “desmosine,” which provides both strength and elasticity. Illustration of function of elastin fibers provides strength and elasticity to a number of tissues (see text). 13-4A. [Reproduced with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.] 13-4B. [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
Elastic fibers are found in several tissue types, but mainly in skin, the outer ear, larynx and epiglottis, blood vessels, lungs, bladder, some intervertebral discs, and as an attachment between teeth and the underlying bones. As the name suggests, elastic fibers can be easily stretched (up to 1.5× their original length). Diseases related to defects in elastin include Menkes disease, Hurler disease, William’s syndrome, cutis laxa, Buschke–Ollendorf syndrome, and pseudoxanthoma elasticum. Changes in the elastic fibers in the heart and/or arteries may also play a role in high blood pressure, as the ability to absorb the force of heart contraction with appropriate blood vessel stretch and rebound is diminished.
Diseases of connective tissues are usually associated with defects in the component collagen molecule or deficiencies in essential nutrients required for their synthesis (e.g., vitamin C) and secretion. A large group of autoimmune diseases of connective tissue also exists, in which the body’s immune system mistakenly attacks parts of the body. If too much collagen is produced, scleroderma may result, an autoimmune disease characterized by thickening and hardening of the skin and deleterious changes in blood vessels. Most of the connective tissue diseases are diagnosed by physical examination, looking for abnormalities in the organ(s) involved that reflect changes in the connective tissue. DNA studies and biopsies can help confirm the condition(s). Autoimmune connective tissue diseases are normally diagnosed by symptoms and by blood tests revealing diagnostic antibodies or reactive molecules. The collagen types, functions, and related diseases are reviewed in Table 13-1.
Type | Location/Functions | Associated Diseases |
|---|---|---|
I (two subtypes) | Found in skin, tendons, ligaments, blood vessels, muscles, various organs, bones, and teeth. Forms scar tissue during wound healing. Most abundant form of collagen. | Ehlers–Danlos syndrome (types I, II, and VII A and B), osteogenesis imperfecta (types I–IV), Caffey’s disease (infantile cortical hyperostosis), atypical Marfan syndrome, possible association with osteoporosis |
II (one subtype) | Hyaline cartilage (major component of this type), vitreous humor (eye) | Types II and XI collagenopathies [achondrogenesis type 2, hypochondrogenesis, Kniest dysplasia, otospondylomegaepiphyseal dysplasia, spondyloepimetaphyseal dysplasia (Strudwick type), spondyloepiphyseal dysplasia congenita, spondyloperipheral dysplasia, Stickler syndrome, Weissenbacher–Zweymüller syndrome, platyspondylic lethal skeletal dysplasia (Torrance type)] |
III (one subtype) | Skin, muscle, intestines, liver, lung, bone marrow, blood vessels, lymphatic system, lens of eyes, and uterus. Also, main component of granulation tissue and reticular fibers. | Ehlers–Danlos syndrome (types III and IV), aneurysms (aortic and arterial) |
IV (six subtypes) | Forms sheets versus fibers. Major component of all cell basement membranes (basal lamina) | Alport syndrome, HANAC (hereditary angiopathy with nephropathy, aneurysms, and muscle cramps), familial benign hematuria, and Goodpasture’s syndrome |
V (three subtypes) | Surface of cells, most interstitial tissues, placenta and other fetal tissues, and hair. Often found with type I. | Ehlers–Danlos syndrome (types I–III) |
VI (three subtypes) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Often associated with type I in extracellular matrix of most interstitial tissues and microfibrils. | Bethlehem myopathy, Ullrich scleroatonic muscular dystrophy |
VII (one subtype) | Component of basement membrane (basal lamina). Anchors stratified squamous epithelial to underlying supportive framework tissue (stroma). Also found in retina. | Dystrophic epidermolysis bullosa and epidermolysis bullosa acquisita |
VIII (two subtypes) | Component of basement membrane (basal lamina) of the corneal endothelium. | Posterior polymorphous dystrophy, type 2 |
IX (three subtypes) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Along with type II, major component of hyaline cartilage. Also, associates with type XI. Also found in vitreous humor. | Early-onset arthritis, epiphyseal dysplasia, Stickler syndrome (recessive variant) |
X (one subtype) | Product of endochondral ossification (see below) | Schmid-type metaphyseal chondrodysplasia (SMCD) and Japanese-type spondylometaphyseal dysplasia (SMD) |
XI (two subtypes) | Muscle, joints, various organs, skin, nose, inner ear and earlobe, vitreous humor, and nucleus pulposus of vertebral discs | Inherited deafness, types II and XI collagenopathies (see a full list under type II), and Marshall syndrome |
XII (one subtype) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Often associated with type I. Found in extracellular matrix of interstitial tissues, embryonic tissue, skin, and growth plate | May play a role in the development of atherogenesis |
XIII (one subtype) | Found in low levels in various connective tissues and, unlike most collagens, appears to be a plasma membrane-bound form. Function unknown, but binds with integrins, fibronectin, and basement membrane (basal lamina). | Unknown |
XIV (one subtype) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Believed to be associated with the extracellular matrix. | Unknown |
XV (one subtype) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Found in multiple tissues, but mainly adheres basement membrane (basal lamina) to underlying structures (stroma). | Defects may cause breakdown of muscle and/or small blood vessels. |
XVI (one subtype) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Often associated with types I and II in extracellular matrix of smooth muscles and amniotic sac membrane (amnion). Also found in fibroblasts and keratinocytes. | Unknown |
XVII (one subtype) | Unlike most collagens, appears to be a plasma membrane-bound form, playing an important part in the structure of hemidesmosomes, adhering epidermal keratinocytes to the underlying basement membrane. Reportedly binds to keratin, α-actinin, and dystonin among others. | Dysfunction leads to condition of junctional epidermolysis bullosa with easy skin blistering, skin, mucus membrane and nail breakdown, hair loss, and adverse changes in teeth structure. May also cause bullous pemphigoid with intermittently formed blisters (bullae) of skin and mucus membranes. |
XVIII (one subtype) | Unlike most collagens, contains collagen-like and non–collagen-like domains. Found in extracellular matrix. Cleavage of the protein to a 20 kDa, C-terminal fragment produces the protein endostatin, which is a strong inhibitor of the migration and proliferation of endothelial cells for the growth of new blood vessels. Mechanism may be by inhibition of growth factors. | Knobloch syndrome with retinal and neural tube structural abnormalities. Endostatin derivative has shown marked effectiveness in cancer treatments (e.g., endocrine, carcinoid, and non-small cell lung carcinoma). |
XIX (one subtype) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. Function unknown, but known to associate with types I and II as part of extracellular matrix structure. | Unknown |
XX (one subtype) | Function unknown, but believed to be involved in adhesion in the extracellular matrix. | Unknown |
XXI (one subtype) | “Fibril-associated collagens” with regularly appearing globular domains giving a “beaded filament” appearance. May assist in assembly of extracellular matrix of smooth muscle cells in heart (right side > left side), blood vessels (e.g., aorta), trachea, stomach, jejunum, colon, liver, pancreas, kidney, testis, uterus and placenta, and lymph nodes. | Unknown |
XXII (one subtype) | Found in skeletal muscle and heart, where it appears to play a yet unknown function in junctions between heart cells and between muscles and tendons. | Unknown |
XXIII (one subtype) | Unlike most collagens, is found in the plasma membrane of lungs, cornea, brain, skin, tendons, and kidney, and contains collagen-like and non-collagen-like domains. Appears to be involved in binding of epithelial cells to basement membrane (basal lamina) possibly via type IV. | Unknown |
XXIV (one subtype) | Found in developing bone and eye usually with type I. Function unknown, but may be involved in collagen expression during fetal development. | Unknown |
XXV (one subtype) | Unlike most collagens, is found in the plasma membrane of brain. Function unknown, but may regulate collagen fibril elongation. | Found in senile plaques in Alzheimer disease |
XXVI (one subtype) | Found in myoid and pre-theca cells in testis and ovary. Function unknown. |