Overview
The female and male reproductive systems develop selectively as a result of specific hormonal signals (sex-determining region on the Y chromosome, anti-Mullerian hormone, testosterone/5α-dihydrotestosterone, and estrogens) that lead to further development or regression of embryological structures. Additional hormones (gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, progesterone, and human chorionic gonadotropin) influence further development and subsequent adult functions, including the menstrual cycle, fertilization and pregnancy, lactation, and oogenesis/spermatogenesis. These hormones work via signaling proteins, including several variations of G proteins, to selectively activate or inhibit these developmental and functional events.
Basic Anatomy and Development
The reproductive system is derived from the intermediate mesoderm and includes the reproductive organs of both males and females derived from the Wolffian ducts (male), Mullerian ducts (female), and the gonads (male and female), including the testes and ovaries. The influence of hormones and biochemical signals in the formation and activity of the reproductive system is vastly important and, when these signals go awry, disease ensues.
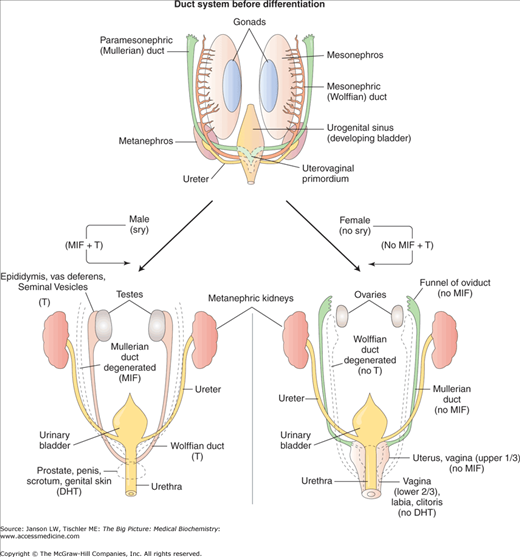
During the initial stages of gestation, all humans begin with both Wolffian and Mullerian ducts and the development of male and female embryos is indistinguishable. At about gestational day 56, further growth and development into male or female sexual organs is dependent on the effects of the sex-determining region of the Y chromosome (sry). In the genetic male, the sry product binds to deoxyribonucleic acid (DNA) and distorts it dramatically out of shape. This alters the properties of the DNA and likely alters the expression of a number of genes. One of these genes produces anti-Mullerian hormone (AMH), a dimeric, glycoprotein hormone also known as Mullerian inhibiting factor (MIF). AMH is produced by Sertoli cells in the testes and signals, via its receptor, a member of the transforming growth factor (TGF)-β I and II receptor family. Binding of AMH to TGF-β type II receptor allows it to bind to the type I receptor. The type I receptor is then able to phosphorylate serine and/or threonine amino acids to activate transcription factors in the nucleus, which regulate gene expression. The presence of AMH leads to full development of the Wolffian ducts and male structures; only a few remnants of the Mullerian ducts survive in males.
In males, the Leydig cells also appear and testosterone synthesis begins, leading to male sexual characteristics, including testis formation. Testosterone’s effect on the seminiferous tubules (see below) is also critical for modulating signaling and gene expression and, therefore, male development (Figure 20-1). In the absence of sry, embryos spontaneously develop into phenotypic females. With no sry product, AMH and testosterone are not produced and the Wolffian ducts regress with only a few remnants remaining. The mechanism of AMH suppression of Mullerian duct formation is unknown. Further development of the Mullerian ducts creates the female sexual organs and structures. In both sexes, the Wolffian duct is responsible for development of the bladder trigone.
Figure 20-1.
Summary of Hormonal Involvement in Sexual Differentiation. DHT, dihydrotestosterone; MIF, Mullerian inhibiting factor; sry, sex-determining region of Y chromosome; T, testosterone; 5αR, 5α-reductase-2. [Adapted with permission from Kibble JD and Halsey CR: The Big Picture: Medical Physiology, 1st edition, McGraw-Hill, 2009.]
Androgen Insensitivity Syndrome (AIS): AIS, also previously known as “testicular feminization,” results from a mutation on the X chromosome, which yields a defective androgen receptor. Because this is an autosomal recessive condition, all patients are genetic males and, therefore, produce sry, AMH, and testosterone/5α-dihydrotestosterone (DHT) normally. However, the defective androgen receptors do not allow the normal androgenic functions to be expressed, leading to failure of Wolffian duct development and complete feminization of the external genitalia and blind ended vagina. These patients are, therefore, phenotypically female with a male karyotype. A similar medical condition, 17α-hydroxylase deficiency, results in the inability to form any androgens (Chapter 3) and, therefore, estrogens as well. The condition, also referred to as “sexual infantilism,” leads to underdevelopment of the gonads (hypogonadism). |
Female Reproductive System
As noted above, the development and function of the female reproductive system is under the influence of several hormone signals. Following development, primary hormones in the female system are follicle-stimulating hormone (FSH), luteinizing hormone (LH), and the hormone that regulates their expression—gonadotropin-releasing hormone (GnRH). These hormones are also essential in the male reproductive system (see below). GnRH production and release begins at puberty and remains active during the male and female reproductive years.
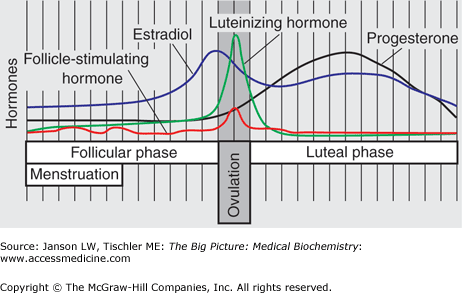
GnRH is a small peptide produced in the hypothalamus by specialized nerve cells; as such, GnRH is called a neurohormone, a class of hormones that include thyrotropin-releasing hormone, oxytocin (see below), antidiuretic hormone (Chapter 18), and corticotropin-releasing hormone. Release of GnRH results in activation of a specific GnRH receptor, located in the gonadotropes of the pituitary gland. This receptor is a membrane-bound G-protein-coupled stimulator of phospholipase C, which results in calcium release and protein kinase C activation via conversion of plasma membrane phosphatidylinositol into inositol triphosphate and diacylglycerol (Chapter 8). These signals result in production and release of FSH and LH, as will be described below. Regulation of this important signal is multifold. GnRH is degraded within minutes so it is constantly produced in pulses, and the size and frequency of these pulses is important in signaling. These GnRH pulses are constant in males but vary in females, depending on the menstrual cycle. Interestingly, the frequency of the GnRH pulses result in different expression of FSH (low frequency) and LH (high frequency). As a result FSH and LH are variably expressed during the female menstrual period (Figure 20-2). Levels of testosterone, estrogen, and prolactin (increased during pregnancy) as well as increased concentration of FSH and LH create a negative feedback loop, which can decrease GnRH pulses.
Figure 20-2.
Variable Expression of Reproductive Hormones During the Menstrual Cycle (Follicle-Stimulating Hormone, Luteinizing Hormone, Estradiol, and Progesterone). Periods of the menstrual cycle, including menstruation, follicular phase, ovulation, and luteal phase are indicated. [Adapted with permission from Kibble JD and Halsey CR: The Big Picture: Medical Physiology, 1st edition, McGraw-Hill, 2009.]
FSH is a dimeric glycoprotein hormone produced and secreted by the anterior pituitary gland. FSH has an α- and β-subunit, with the α-subunit being identical in amino acid sequence to the α-subunit of LH, thyroid-stimulating hormone (TSH), and human chorionic gonadotropin (hCG) (see below). The β-subunit binds to and activates the FSH receptor.
The FSH receptor is believed to exist in two conformational states (Chapter 1)—one active and the other inactive. Binding of FSH to its receptor locks the receptor’s conformation into the active form, which activates a Gs-coupled protein causing release of an αs-subunit. This αs-subunit then activates adenyl cyclase to increase the cyclic adenosine monophosphate (cAMP) signal and protein kinase A activity (Chapter 8). Phosphorylated signaling proteins bind to cAMP response elements on DNA, leading to activation of particular genes. FSH can also activate the family of extracellular signal-related kinases. This family of receptor-activated kinase, also known as mitogen-activated protein (MAP) kinases, respond to a variety of signal molecules, including FSH, which regulate meiosis and mitosis of selected cells. Although many mechanisms may be in play, the activation of tyrosine kinases via the ras GTPase protein is considered important in the subsequent activation of transcription factors.
FSH performs many functions in the development of female and male and is also essential for the proper timing and phasing of the menstrual cycle. FSH, as its name implies, stimulates granulosa cells in the follicles in the ovary to initiate egg growth and development (oogenesis). FSH specifically blocks programmed death or atresia of early developing egg follicles. As one egg follicle becomes dominant, reaching about 10 μm in diameter, it begins to secrete estradiol, which negatively feedbacks on GnRH and, therefore, FSH production. The block of atresia in all follicles except the dominant one leads to the survival of only one egg.
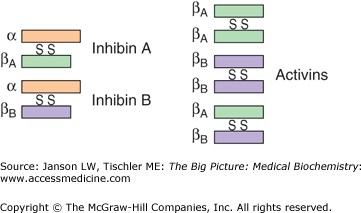
The activity of FSH is regulated by several means. FSH has a biological half-life of only about 3–4 hrs. Unless GnRH pulses continue to promote FSH synthesis and secretion, its biochemical effects will begin to diminish. Increased estrogen will also decrease FSH synthesis via its effect on GnRH. Activin (Figure 20-3, right), a protein heterodimer (composed of two different subunits) or homodimer (composed of two identical subunits), serves to increase FSH production and secretion via a mechanism extremely similar to the FSH receptor, leading to protein kinase A activity and upregulation of gene expression. However, the same small antral follicles that FSH stimulates also produce the protein inhibin (Figure 20-3, left), a protein heterodimer with one subunit identical to the activin subunit and one different. Inhibin decreases FSH synthesis and secretion and, therefore, works in a similar manner to the dominant follicle’s estrogen production to decrease FSH and increase nondominant follicle atresia.
Figure 20-3.
Inhibins and Activins. Illustration of structures of inhibins (left), heterodimers of three separate proteins (represented as three different colors), and activins (right), homodimers or heterodimers of the green and purple proteins. [Adapted with permission from Barrett KE, et al.: Ganong’s Review of Medical Physiology, 23rd edition, McGraw-Hill, 2010.]
As the luteal phase ends, a small rise in FSH can be detected that helps to signal the start of the next menstrual cycle. In addition, estrogen and progesterone levels fall and decrease their negative effect on GnRH, allowing FSH levels to rise with an initial peak at day 3 (Figure 20-2). In a directly opposite signaling mechanism, estrogen increases GnRH and, therefore, FSH secretion at the time of ovulation (see below). Finally, as a woman approaches menopause, the number of initial follicles recruited to egg production decreases and, as a result, the level of inhibin is also lessened. This leads to an overall rise in serum FSH levels that is one sign of perimenopause/menopause.
LH is a dimeric glycoprotein, composed of an α- and β-subunit, produced by the anterior pituitary gland. The α-subunit is identical in amino acid sequence to the α-subunit of FSH, TSH, and hCG (see below). The β-subunit is similar but not identical to that of hCG and is responsible for binding to the LH receptor. LH is not produced until puberty and then is generated in response to GnRH signals, regulated according to reproductive need. Like FSH, LH levels also rise in females after menopause.
Like the FSH receptor, the LH receptor is believed to exist in two conformational states (Chapter 1)—one active and the other inactive. Binding of LH to its receptor locks the receptor’s conformation into the active form, which activates a Gs-coupled protein causing release of an αs-subunit. This αs-subunit then activates adenyl cyclase to produce the cAMP signal and increased protein kinase A activity (Chapter 8). Phosphorylated signaling proteins bind to cAMP response elements on DNA, leading to activation of particular genes. LH has a biological half-life of about 20 min, so gene regulation via its receptor may stop unless additional LH or other signaling molecules continue the nuclear signal.
In females, LH provides the hormone signal for release of an egg from the ovary. Increasing estrogen, which results from FSH stimulation of ovarian granulosa cells, increases the presence of LH receptors. In addition, estrogen activates the pituitary to increase LH secretion. Both of these functions act to produce an LH “surge” over a 24–48 hr period (Figure 20-2). Unlike FSH, activin, inhibin, and the sex hormones do not affect LH production at the DNA level. The LH surge produces ovulation and also signals the corpus luteum, a temporary structure created from the remnant ovarian follicle after egg release. The activated corpus luteum produces the hormone progesterone, which, in turn, signals the uterine wall to prepare for egg implantation if fertilization should occur. The presence of LH also starts and, in the case of fertilization and implantation of the egg, maintains the luteal phase (Figure 20-2) for 8 weeks. LH promotes androgen and estrogen production via stimulation of thecal cells in the ovary. The LH surge also initiates the continuation of meiosis in the oocyte, the completion of which occurs after the sperm enters the ovum. Finally, LH is important to luteinize the granulosa cells to produce progesterone for maintenance of the luteal phase.
Ovulation Prediction by LH Measurement: The 24–48 hr LH surge that leads to ovulation and, therefore, predicts an optimal time for conception has led to the development of “ovulation predictor kits” to assist couples wanting children. These kits have anti-LH antibodies, which bind to LH excreted in the urine. Binding of the LH and a positive color change via a chemical reaction indicates when the level is above the normal 1–20IU/L—levels indicating maximum fertility times. Digital measurements of LH level are also available. |
Estrogen is the inclusive term for a group of steroids that primarily impact the female reproductive system. Estrogens are mainly synthesized when FSH and LH stimulate thecal and granulosa cells in the ovarian follicles, the corpus luteum after release of a dominant egg, and, in the case of pregnancy, the placenta. The liver, adrenal glands, adipose tissue, and breast tissue can also produce steroidal estrogen. The highest level of estrogen occurs immediately prior to ovulation.
As a class of steroid hormones, all estrogens function by crossing the cell membrane and activating estrogen receptors (ERs). Unbound ERs are mainly found in the cytosol but, upon binding of an estrogen molecule, the receptors move into the nucleus, form dimers, and bind to specific sequences of the DNA molecule known as hormone responsive elements. The bound ER–DNA activates particular proteins that start transcription of DNA to result in synthesis of specific proteins. ERs can also be found in the nucleus where they serve to regulate the transcription of other proteins and may also associate with plasma membrane G proteins, which activate tyrosine kinases. Three major forms of estrogen molecules occur in humans (Figure 20-4 and Chapter 3).