Key Concepts
 Study designs in medicine fall into two categories: studies in which subjects are observed, and studies in which the effect of an intervention is observed.
Study designs in medicine fall into two categories: studies in which subjects are observed, and studies in which the effect of an intervention is observed.
 Observational studies may be forward-looking (cohort), backward-looking (case-control), or looking at simultaneous events (cross-sectional). Cohort studies generally provide stronger evidence than the other two designs.
Observational studies may be forward-looking (cohort), backward-looking (case-control), or looking at simultaneous events (cross-sectional). Cohort studies generally provide stronger evidence than the other two designs.
 Studies that examine patient outcomes are increasingly published in the literature; they focus on specific topics, such as resource utilization, functional status, quality of life, patient satisfaction, and cost-effectiveness.
Studies that examine patient outcomes are increasingly published in the literature; they focus on specific topics, such as resource utilization, functional status, quality of life, patient satisfaction, and cost-effectiveness.
 Studies with interventions are called experiments or clinical trials. They provide stronger evidence than observational studies.
Studies with interventions are called experiments or clinical trials. They provide stronger evidence than observational studies.
 The single best way to minimize bias is to randomly select subjects in observational studies or randomly assign subjects to different treatment arms in clinical trials.
The single best way to minimize bias is to randomly select subjects in observational studies or randomly assign subjects to different treatment arms in clinical trials.
 Bias occurs when the way a study is designed or carried out causes an error in the results and conclusions. Bias can be due to the manner in which subjects are selected or data are collected and analyzed.
Bias occurs when the way a study is designed or carried out causes an error in the results and conclusions. Bias can be due to the manner in which subjects are selected or data are collected and analyzed.
 Clinical trials without controls (subjects who do not receive the intervention) are difficult to interpret and do not provide strong evidence.
Clinical trials without controls (subjects who do not receive the intervention) are difficult to interpret and do not provide strong evidence.
 Each study design has specific advantages and disadvantages.
Each study design has specific advantages and disadvantages.
Study Designs in Medical Research: Introduction
This chapter introduces the different kinds of studies commonly used in medical research. Because we believe that knowing how a study is designed is important for understanding the conclusions that can be drawn from it, we have chosen to devote considerable attention to the topic of study designs.
If you are familiar with the medical literature, you will recognize many of the terms used to describe different study designs. If you are just beginning to read the literature, you should not be dismayed by all the new terminology; there will be ample opportunity to review and become familiar with it. Also, the glossary at the end of the book defines the terms we use here. In the final chapter of this book, study designs are reviewed within the context of reading journal articles, and pointers are given on how to look for possible biases that can occur in medical studies. Bias can be due to the manner in which patients are selected, data are collected and analyzed, or conclusions are drawn.
Classification of Study Designs
![]() There are several different schemes for classifying study designs. We have adopted one that divides studies into those in which the subjects were merely observed, sometimes called observational studies, and those in which some intervention was performed, generally called experiments. This approach is simple and reflects the sequence an investigation sometimes takes. With a little practice, you should be able to read medical articles and classify studies according to the outline in Table 2–1 with little difficulty.
There are several different schemes for classifying study designs. We have adopted one that divides studies into those in which the subjects were merely observed, sometimes called observational studies, and those in which some intervention was performed, generally called experiments. This approach is simple and reflects the sequence an investigation sometimes takes. With a little practice, you should be able to read medical articles and classify studies according to the outline in Table 2–1 with little difficulty.
| I. Observational studies |
| A. Descriptive or case–series |
| B. Case–control studies (retrospective) |
| 1. Causes and incidence of disease |
| 2. Identification of risk factors |
| C. Cross-sectional studies, surveys (prevalence) |
| 1. Disease description |
| 2. Diagnosis and staging |
| 3. Disease processes, mechanisms |
| D. Cohort studies (prospective) |
| 1. Causes and incidence of disease |
| 2. Natural history, prognosis |
| 3. Identification of risk factors |
| E. Historical cohort studies |
| II. Experimental studies |
| A. Controlled trials |
| 1. Parallel or concurrent controls |
| a. Randomized |
| b. Not randomized |
| 2. Sequential controls |
| a. Self-controlled |
| b. Crossover |
| 3. External controls (including historical) |
| B. Studies with no controls |
| III. Meta-analyses |
Each study design in Table 2–1 is illustrated in this chapter, using some of the studies that are presenting problems in upcoming chapters. In observational studies, one or more groups of patients are observed, and characteristics about the patients are recorded for analysis. Experimental studies involve an intervention—an investigator-controlled maneuver, such as a drug, a procedure, or a treatment—and interest lies in the effect the intervention has on study subjects. Of course, both observational and experimental studies may involve animals or objects, but most studies in medicine (and the ones discussed most frequently in this text) involve people.
Observational Studies
![]() Observational studies are of four main types: case–series, case–control, cross-sectional (including surveys), and cohort studies. When certain characteristics of a group (or series) of patients (or cases) are described in a published report, the result is called a case–series study; it is the simplest design in which the author describes some interesting or intriguing observations that occurred for a small number of patients.
Observational studies are of four main types: case–series, case–control, cross-sectional (including surveys), and cohort studies. When certain characteristics of a group (or series) of patients (or cases) are described in a published report, the result is called a case–series study; it is the simplest design in which the author describes some interesting or intriguing observations that occurred for a small number of patients.
Case–series studies frequently lead to the generation of hypotheses that are subsequently investigated in a case–control, cross-sectional, or cohort study. These three types of studies are defined by the period of time the study covers and by the direction or focus of the research question. Cohort and case–control studies generally involve an extended period of time defined by the point when the study begins and the point when it ends; some process occurs, and a certain amount of time is required to assess it. For this reason, both cohort and case–control studies are sometimes also called longitudinal studies. The major difference between them is the direction of the inquiry or the focus of the research question: Cohort studies are forward-looking, from a risk factor to an outcome, whereas case–control studies are backward-looking, from an outcome to risk factors. The cross-sectional study analyzes data collected on a group of subjects at one time. Kleinbaum and colleagues (1997) describe a number of hybrids or combinations of these designs if you are interested in more detail than we give in this chapter. If you would like a more detailed discussion of study designs used in medicine, see the companion text on epidemiology by Greenberg and coworkers (2000). A book by Hulley and Cummings (2001) is devoted entirely to the design of clinical research. Garb (1996) and Burns and Grove (2002) discuss study design in medicine and nursing, respectively.
A case–series report is a simple descriptive account of interesting characteristics observed in a group of patients. For example, Alexandrov and coworkers (1997) presented information on a series of 40 patients who had been referred for evaluation of stroke, transient ischemic attack, or carotid bruit. The authors wanted to compare two methods to see which better predicted peak systolic velocity. They concluded that the relationship between both methods and peak systolic velocity was very strong.
Case–series reports generally involve patients seen over a relatively short time. Generally case–series studies do not include control subjects, persons who do not have the disease or condition being described. Some investigators would not include case–series in a list of types of studies because they are generally not planned studies and do not involve any research hypotheses. On occasion, however, investigators do include control subjects. We mention case–series studies because of their important descriptive role as a precursor to other studies.
Case–control studies begin with the absence or presence of an outcome and then look backward in time to try to detect possible causes or risk factors that may have been suggested in a case–series report. The cases in case–control studies are individuals selected on the basis of some disease or outcome; the controls are individuals without the disease or outcome. The history or previous events of both cases and controls are analyzed in an attempt to identify a characteristic or risk factor present in the cases’ histories but not in the controls’ histories.
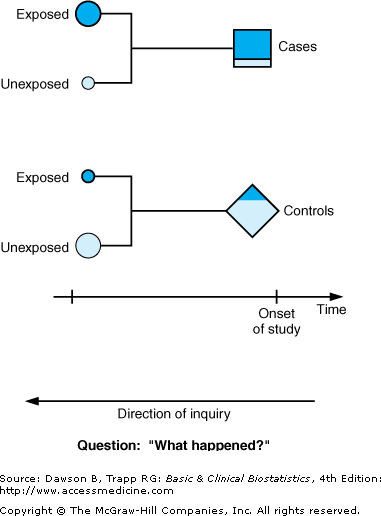
Figure 2–1 illustrates that subjects in the study are chosen at the onset of the study after they are known to be either cases with the disease or outcome (squares) or controls without the disease or outcome (diamonds). The histories of cases and controls are examined over a previous period to detect the presence (shaded areas) or absence (unshaded areas) of predisposing characteristics or risk factors, or, if the disease is infectious, whether the subject has been exposed to the presumed infectious agent. In case–control designs, the nature of the inquiry is backward in time, as indicated by the arrows pointing backward in Figure 2–1 to illustrate the backward, or retrospective, nature of the research process. We can characterize case–control studies as studies that ask “What happened?” In fact, they are sometimes called retrospective studies because of the direction of inquiry. Case–control studies are longitudinal as well, because the inquiry covers a period of time.
Figure 2–1.
Schematic diagram of case–control study design. Shaded areas represent subjects exposed to the antecedent factor; unshaded areas correspond to unexposed subjects. Squares represent subjects with the outcome of interest; diamonds represent subjects without the outcome of interest. (Adapted and reproduced, with permission, from Greenberg RS: Retrospective studies. In Kotz S, Johnson NL [editors]: Encyclopedia of Statistical Sciences, Vol 8. Wiley, 1988.)
Olsen and colleagues (2003) compared patients who had a surgical site infection following laminectomy or spinal fusion (cases) with patients who developed no infection (controls). The investigators found that length of hospital stay and readmission rates were greater with patients with infections. Furthermore, postoperative incontinence was one of the risk factors associated with the development of infection.
Investigators sometimes use matching to associate controls with cases on characteristics such as age and sex. If an investigator feels that such characteristics are so important that an imbalance between the two groups of patients would affect any conclusions, he or she should employ matching. This process ensures that both groups will be similar with respect to important characteristics that may otherwise cloud or confound the conclusions.
Deciding whether a published study is a case–control study or a case–series report is not always easy. Confusion arises because both types of studies are generally conceived and written after the fact rather than having been planned. The easiest way to differentiate between them is to ask whether the author’s purpose was to describe a phenomenon or to attempt to explain it by evaluating previous events. If the purpose is simple description, chances are the study is a case–series report.
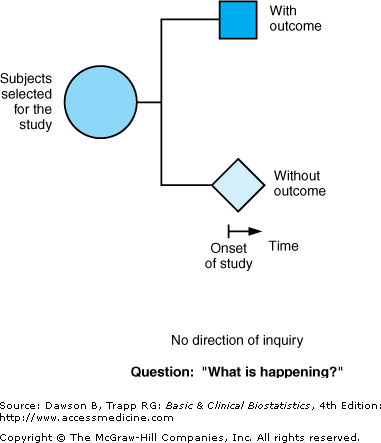
The third type of observational study goes by all of the following names: cross-sectional studies, surveys, epidemiologic studies, and prevalence studies. We use the term “cross-sectional” because it is descriptive of the time line and does not have the connotation that the terms “surveys” and “prevalence” do. Cross-sectional studies analyze data collected on a group of subjects at one time rather than over a period of time. Cross-sectional studies are designed to determine “What is happening?” right now. Subjects are selected and information is obtained in a short period of time (Figure 2–2; note the short time line). Because they focus on a point in time, they are sometimes also called prevalence studies. Surveys and polls are generally cross-sectional studies, although surveys can be part of a cohort or case–control study. Cross-sectional studies may be designed to address research questions raised by a case–series, or they may be done without a previous descriptive study.
In a presenting problem in Chapter 10, Soderstrom and his coinvestigators (1997) were interested in learning more about the relationship between demographic measures that might be helpful in identifying trauma patients who have an elevated blood alcohol concentration. They wanted to develop a simple scoring system that could be used to detect these patients when they come to an emergency department. These patients could be targeted for assessment of alcohol abuse and dependence and other possible substance abuse. They chose to look at the time of day (day or night), the day of the week (weekday or weekend), race (white or nonwhite), and age (40 years or older versus younger than 40). Using these four simple measures, the investigators were able to construct four models: for men whose injury was intentional, men whose injury was not intentional, women whose injury was intentional, and women whose injury was not intentional.
A presenting problem in Chapter 5 is a cross-sectional study designed to examine the relationship between histology slides and magnetic resonance imaging (MRI) to study characteristics of diseased carotid arteries (Yuan et al, 2001). The histology slides were evaluated by a pathologist who was blinded to the imaging results. It is important to establish the level of agreement between the MRI findings and histology, and the level of agreement was found to be relatively high. Cross-sectional studies are used in all fields of medicine, but they are especially common in examinations of the usefulness of a new diagnostic procedure.
Knowledge of the range within which most patients fit is very useful to clinicians. Laboratories, of course, establish and then provide the normal limits of most diagnostic tests when they report the results for a given patient. Often these limits are established by testing people who are known to have normal values. We would not, for example, want to use people with diabetes mellitus to establish the norms for serum glucose levels. The results from the people known to have normal values are used to define the range that separates the lowest 2½% of the values and the highest 2½% of the values from the middle 95%. These values are called normal values, or norms.
Outside of the laboratory there are many qualities for which normal ranges have not been established. This was true for two measures of the autoimmune nervous system function. These two measures, heart variation to deep breathing and the Valsalva ratio, are noninvasive tests that can help clinicians evaluate patients with diabetes mellitus and other neuropathic disorders. Gelber and colleagues (1997) analyzed data from subjects recruited from 63 centers throughout North America to develop normative values for these two measurements. After comparing certain demographic groups, such as males versus females, the investigators established the normative values for heart rate variation to deep breathing and the Valsalva.
Surveys are especially useful when the goal is to gain insight into a perplexing topic or to learn how people think and feel about an issue. Surveys are generally cross-sectional in design, but they can be used in case–control and cohort studies as well.
Caiola and Litaker (2000) wanted to know the factors that influence fellows to select a specific general internal residency fellowship program. Because they did not know the names and addresses of the fellows, the authors sent a questionnaire to the program directors and asked them to distribute the questionnaires to the fellows. We examine this study in more detail in Chapter 11 and illustrate how the authors asked the questions on the survey.
Many times investigators use preexisting surveys rather than creating their own, especially if good questionnaires already exist. Patenaude and colleagues (2003) asked medical students at a Canadian medical school to complete a questionnaire on moral reasoning (the Kohlberg Moral Judgment Interview). They wanted to learn how moral reasoning progressed over time, so they gave the questionnaire at the beginning of medical school and again at the end of the third year. They learned that the stage of moral development did not change in about 70% of the students, whereas it either decreased or increased in 15%. The authors had expected the level of moral reasoning to increase, and the results of the study prompted them to raise questions about the possible features of medical education that might inhibit its development.
Interviews are sometimes used in surveys, especially when it is important to probe reasons or explanations more deeply than is possible with a written questionnaire. Kendler and colleagues (2003) wanted to investigate the role of genetic and environmental risk factors for substance abuse. They studied six classes of illicit substances to learn whether substance use disorders are substance-specific. After interviewing almost 1200 sets of adult male twins, they concluded that environmental experiences unique to a given individual are primarily responsible for whether the person misuses one class of psychoactive substances over another. Increasingly, surveys are performed using existing databases of information. As an illustration, Huang and Stafford (2002) used survey data from the National Ambulatory Medical Care Survey to examine the relationship between demographics and clinical characteristics of women who visit primary care physicians and specialists for urinary tract infection. Using preexisting databases can have a number of advantages, such as saving time and effort, but many national surveys use complicated designs; and it is important to know what these are, as we discuss when we explore this study in more detail in Chapter 11.
Many countries and states collect data on a variety of conditions to develop tumor registries and databases of cases of infectious disease. Diermayer and colleagues (1999), a presenting problem in Chapter 4, analyzed epidemiologic surveillance data from the State of Oregon and reported an increase in the overall incidence rate of meningococcal disease from 2 cases/100,000 population during 1987–1992 to 4.5 cases/100,000 in 1994. Epidemiologists from Oregon and the Centers for Disease Control in Atlanta, Georgia, wanted to know if the increased number of cases of meningococcal disease indicated a transition from endemic to epidemic disease. They also sought these other features of an epidemic: the predominance of a single bacterial strain rather than a heterogeneous mix of strains and a shift in age distri bution of cases toward older age groups.
A cohort is a group of people who have something in common and who remain part of a group over an extended time. In medicine, the subjects in cohort studies are selected by some defining characteristic (or characteristics) suspected of being a precursor to or risk factor for a disease or health effect. Cohort studies ask the question “What will happen?” and thus, the direction in cohort studies is forward in time. Figure 2–3 illustrates the study design. Researchers select subjects at the onset of the study and then determine whether they have the risk factor or have been exposed. All subjects are followed over a certain period to observe the effect of the risk factor or exposure. Because the events of interest transpire after the study is begun, these studies are sometimes called prospective studies.