Overview
Carbohydrates are vastly important in human biology, including roles as a major energy source, structural molecules when combined with other carbohydrates, proteins, and other molecules, and binding and signaling between molecules and cells. As a result of all these important functions, carbohydrate biochemistry is involved in a large number of disease states. Although multiple carbohydrates exist, only a few sugar molecules and polysaccharides are important to human physiology (e.g. only eight different carbohydrates are found as constituents of glycoproteins and glycolipids). However, a number of additional molecules created by linkages of carbohydrates to proteins play various roles in cell–cell interactions and biological structures.
Basic Carbohydrate Structure and Function
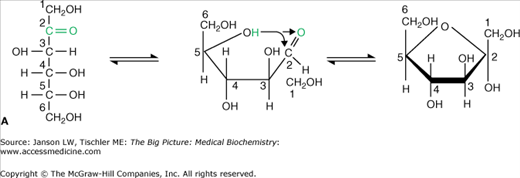
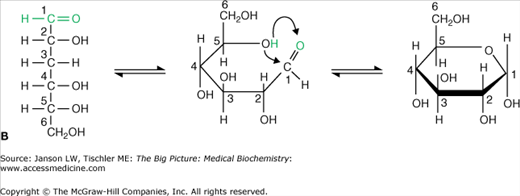
Carbohydrates, whose names end in “-ose,” have a formula of (CH2O)x where x is a number from three to seven (giving the names of triose, tetrose, pentose, hexose, and heptose). All carbohydrates contain a ketone or an aldehyde group, as well as one or more hydroxyl groups (Figure 2-1A–B; Appendix III). The oxygen atoms of the ketone and aldehyde groups have similar reactive qualities to that of the carboxylic acid group seen in amino acids and are the sites of chemical reactions within the carbohydrate molecule, as well as with other carbohydrate, protein, or lipid molecules. Often, the ketone or aldehyde reacts with a hydroxyl group from the same sugar molecule to form a carbohydrate ring structure as shown.
Figure 2-1
A–B. Basic Carbohydrate Structures. A. The reactive ketone group of carbon 2 (green carbon group) from the hexose fructose reacts with the hydroxyl group of carbon 5 to form a new bond and a five-sided (pentose) ring structure. All carbon atoms are numbered for clarity. This reaction is fully reversible as indicated by the bidirectional arrows. As a result, the linear and ring structures are constantly changing in solution. B. The reactive aldehyde group of carbon 1 (green carbon group) from the hexose glucose reacts with the hydroxyl group of carbon 5 to form a new bond and a six-sided (hexose) ring structure. All carbon atoms are numbered for clarity. This reaction is fully reversible as indicated by the bidirectional arrows. As a result, the linear and ring structures are constantly changing in solution. [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
Carbohydrates play a major role in humans as energy sources and storage, and their role in diet and nutrition, although sometimes controversial, is always one of supreme importance. However, carbohydrates play other roles as noted in Table 2-1.
Biochemical Role | Notes | Examples |
|---|---|---|
Energy source | Carbohydrate metabolism | ATP is a phosphorylated derivative of a carbohydrate |
Structural | Nucleic acids Cell wall (bacteria and plants) | DNA (deoxyribonucleic acid) |
Binding | Points of binding between separate molecules | Binding of influenza virus to target cells |
Signaling | Recognition of specific sugars during binding elicits particular biological responses in and between cells | Lymphocyte–monocyte recognition and immune response |
Monosaccharides and Disaccharides
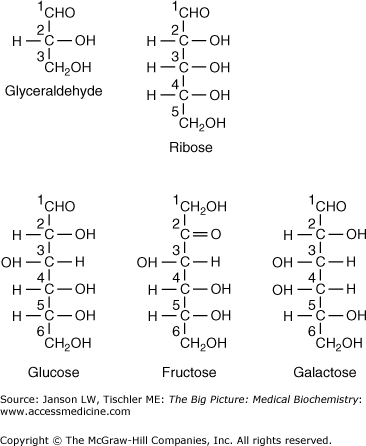
Although there are multiple trioses, pentoses, hexoses and heptoses depending on the various arrangements of hydroxyl groups and hydrogens around the central carbon backbone, only a few of these single residue sugars are commonly seen in human biology. Common single sugar groups, called monosaccharides, include the triose glyceraldehyde; the pentose ribose; and the hexoses fructose, glucose, and galactose (shown in Figure 2-2).
Figure 2-2.
Common Monosaccharides in Human Biology. The common monosaccharide carbohydrates found in humans are shown above, including the three-carbon triose glyceraldehyde; the five-carbon pentose ribose; and the six-carbon hexoses fructose, glucose, and galactose. Note the only structural difference between glucose and galactose is the placement of the hydrogen atom and hydroxyl group at carbon 4. Carbon atoms are numbered for clarity. [Adapted with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
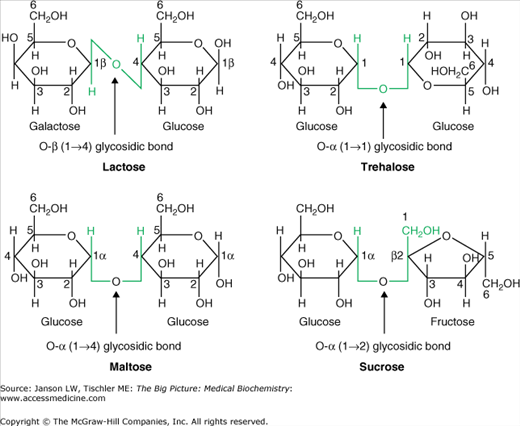
Hydroxyl groups (OH) are often locations of enzyme reactions, especially the formation of a new bond between two carbohydrate molecules with the resulting release of a water molecule. When monosaccharides form such bonds, the resulting molecules are a disaccharide, trisaccharide, and so on. The various combinations of all the different monosaccharides would produce a vast mixture of these new molecules but, in fact, only a few are common in humans (Figure 2-3), namely lactose (the primary sugar found in milk), trehalose [found in plants (e.g., sunflower seeds), animals (e.g., shrimp), Baker’s yeast, and several types of mushrooms)], maltose [(found in many foods made from grains (e.g., barley)], and sucrose (found naturally in plants; usually artificially made for human consumption as common table sugar). The ring structure of monosaccharides also has reactive hydroxyl groups at each of their carbon atoms, especially at the first and sixth carbons, which can bond to other sugar molecules and amino groups and proteins (discussed below and in the following chapters).
Figure 2-3.
Common Disaccharides in Human Biology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree