Overview
The nervous system provides a network of coordinated signaling for bodily functions. Somatic nerves promote skeletal muscle activity and, therefore, gross movements. Autonomic nerves provide signals for internal organs, including regulation of their function. These signals are transmitted by nerves, which rely on membrane-bound protein pumps for conduction of the impulse. Specialized lipids form myelin sheaths, which aid in the fast and efficient transmission of these signals. Neurotransmitters, often small peptides, allow continuation of the nerve signal between neurons and from neurons to target tissues. The breakdown of this network of signaling and regulation leads to several neurological diseases.
Components of the Nervous System
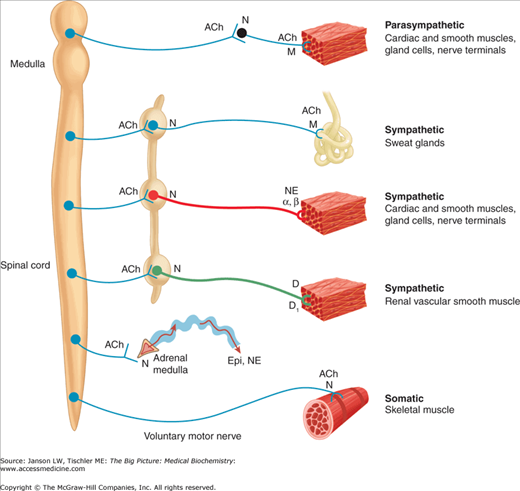
The nervous system is anatomically and functionally composed of two main parts: the central nervous system (CNS) and the peripheral nervous system (PNS) (Figure 19-1). The CNS consists of the brain and spinal cord and the PNS is composed of all nerves outside of the CNS, including all spinal and cranial nerves. In addition, there are further classifications for the nerves of the PNS. First, nerves are distinguished by the direction of nerve propagation. Afferent (sensory) neurons conduct action potentials toward the CNS. Efferent (motor) neurons transmit impulses away from the CNS to effectors such as muscles or glands. Second, nerves of the PNS can be further divided into the somatic (skeletal muscle) and autonomic (organs, glands, and smooth muscle)nervous systems. The autonomic nervous system (ANS) is divided into the sympathetic (SNS) and parasympathetic nervous system (PSNS). Generally, both interact with the same effectors, but often paradoxically. The SNS is involved in the activities associated with the fight-or-flight response, helping the body to cope with stress. The PSNS promotes activities that support the body while at rest, including digestion.
Figure 19-1.
Overview of the Nervous System. Schematic diagram comparing some anatomic and neurotransmitter features of autonomic and somatic motor nerves. Only the primary transmitter substances are shown; parasympathetic ganglia are not indicated. See text for more details. Ach, acetylcholine; D, dopamine; Epi, epinephrine; M, muscarinic receptors; N, nicotinic receptors; NE, norepinephrine. [Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009.]
Neurons are the fundamental components of the nervous system. The main components of a neuron include the soma (or cell body), dendrites, axon, and the axon terminals (Figure 19-2). The cell body, or soma, is the central part of the neuron that contains the nucleus and other cell organelles. The dendrites are the branching structures of the neuron that receives stimuli or messages via chemoreceptors. The axon is a slender, cable-like extension of a neuron that carries nerve impulses away from the soma toward the axon terminal. The axon terminals are the hair-like terminals of the axon where neurotransmitters are released from the synaptic knobs. Neurons communicate with one another via synaptic transmission, where the axon terminal of one neuron comes into close contact with the dendrites of another neuron. Neurons can communicate chemically, via neurotransmitters, or electrically with electrically conductive junctions between the cells.
The myelin sheath is a phospholipid membrane that surrounds and protects the axons of some nerves (Figure 19-2). The primary phospholipid of the membrane is galactocerebroside, a sphingolipid (Chapters 3 and 8). Such phospholipids in nerve membranes strengthen the sheath. Myelin is produced by Schwann cell in the PNS and by oligodendrocytes in the CNS. The neurofibrillar nodes (also known as nodes of Ranvier) are the gaps in the myelin sheath, where the action potential occurs during conduction along the axon.
Figure 19-2.
General Structure of a Nerve Cell. The general structure of a nerve cell includes the cell body with a nucleus and nucleolus, multiple dendrites, and an axon. Further details are provided in the text. The initial segment, immediately after the axon hillock, contains no myelin sheath but does contain a very high density of voltage-gated Na+ channels for continued propagation of the nerve impulse. In the myelinated section, the impulse is conducted from node to node (see below) until reaching the terminal boutons where neurotransmitter molecules are stored for release and nerve signal propagation (see below). [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
Nerve Impulse Conduction
Successful conduction of nerve impulses relies on three elements: an electrically insulated membrane, energy-driven pumps to establish and maintain ion gradients, and ionic selective channels.
Demyelinating Disorders: Demyelinating disorders refer to any condition that disrupts myelin in either the CNS or PNS resulting in an interruption of normal nerve transmission. The process of demyelination can be because of an ischemic, metabolic, genetic, or infectious insult. However, because a demyelinating disorder can follow an infection or an inoculation, it has been hypothesized that an autoimmune mechanism is to blame. In some disorders, remyelination can occur with an associated degree of neural recovery. However, many times, neurologic deficits are permanent. Because myelin is formed in the CNS by oligodendrocytes and in the PNS by Schwann cells, demyelinating disorders can target different locations and have a variable presentation as illustrated below. | Print
|
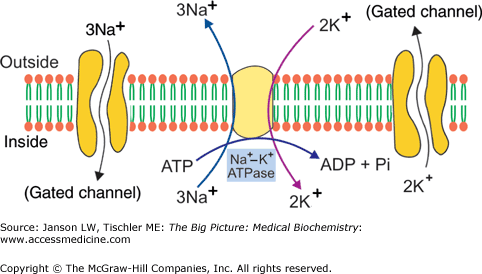
Neurons maintain a resting membrane potential of –70 mV by adenosine triphosphate (ATP)-mediated active transport and passive diffusion of ions. The Na+–K+-ATPase pump of the cell membrane transfers three Na+ out of the cell while transferring two K+ intracellularly (Chapter 8 and Figure 19-3). The steady-state condition results in an extracellular Na+ concentration of 150 mmol/L and an intracellular Na+ concentration of 5–10 mmol/L. The extracellular K+ concentration is 3–4 mmol/L and intracellular is 120–135 mmol/L. This gives rise to the negative state within the cell, and it creates a concentration gradient that will favor the extracellular diffusion of potassium and the intracellular diffusion of sodium upon depolarization.
Figure 19-3.
The Na+–K+-ATPase Pump. The pump and associated sodium (Na+)- and potassium (K+)-gated channels are shown. These gated channels allow the slow restoration of Na+ and K+ to their respective opposite side (see Figure 19-4 and associated text). ADP, adenosine diphosphate; ATP, adenosine triphosphate. [Reproduced with permission from Naik P: Biochemistry, 3rd edition, Jaypee Brothers Medical Publishers (P) Ltd., 2009.]
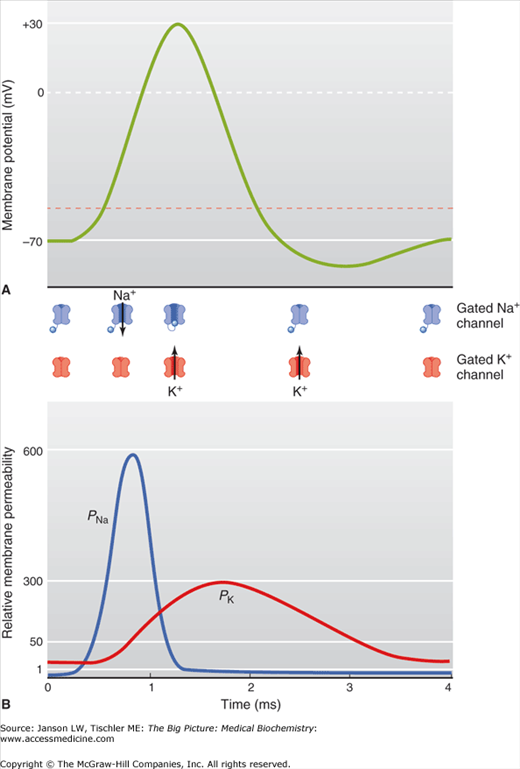
Several stimuli can activate a neuron leading to the generation of a nerve impulse (Figure 19-4). These include chemical, mechanical, or electrical excitation. Impulse propagation is accompanied by small membrane depolarizations. When the depolarization exceeds the threshold level of -55 mV, activated sodium channels cause a flood of Na+ ions into the cell which drives further depolarization. This causes a relative excess of cations in the cell, which eventually leads to a membrane potential of +35 mV. If the initial stimulus is less than −55 mV, few Na+ channels are opened and the threshold level is never reached. As a result, nerve conduction is an all-or-none response.
Figure 19-4.
A–B. The Nerve Impulse. (A) The resting membrane is maintained at a membrane potential of –70 mV. The onset of an action potential opens the gated Na+ channels (see Figure 19-3 and figures below upper graph), leading to the efflux of Na+. Once the threshold level is reached at –55 mV (dashed red line), the depolarization of the membrane caused by Na+ is rapid until a maximum membrane potential of +30–35 mV is reached. At this maximum voltage, the gated Na+ channels close and the gated K+ channels open (see Figure 19-3 and figures below upper graph) and an influx of K+ leads to a drop in the membrane potential. The Na+–K+-ATPase pump (Figure 19-3) also contributes to restoration of the resting membrane potential (not shown). (B) Illustration of relative membrane permeability of Na+ (PNa+) and K+ (PK+) during propagation of the nerve impulse. [Reproduced with permission from Barrett KE, et al.: Ganong’s Review of Medical Physiology, 23rd edition, McGraw-Hill, 2010.]
Niemann–Pick C Disease: Niemann–Pick C Disease is an autosomal recessive, life-threatening lysosomal storage disease, striking mainly in mid-to-late childhood but also in infants and adults. A majority of cases involve a mutation on the NPC1 gene on chromosome 18 resulting in lysosomal cholesterol and glycosphingolipid accumulation in neurons and glial cells in the CNS. The lipid build-up results in a distortion of the neuron mainly by erroneous formation of dendrites, which subsequently alters neurotransmission. The clinical presentation is highly variable but usually presents with the onset of movement and learning disabilities and early impairment of vertical eye movement (vertical supranuclear gaze palsy). In addition, patients can experience loss of muscle tone (dystonia), seizures, disabling joint problems, problems swallowing (dysphagia), and, in adults, varying cognitive impairment as well as psychosis and/or depression. Most patients die due to complications of this disease before the age of 20 years. |
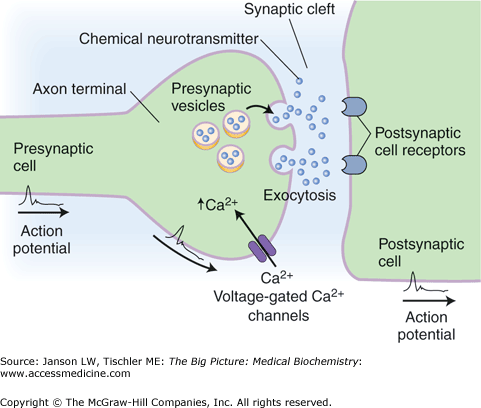
As the neuron’s action potential depolarizes, voltage-gated calcium channels cause an influx of calcium ions (Ca2+). This Ca2+ influx triggers the fusion of storage vesicles with the terminal bud membrane, releasing a neurotransmitter into the synaptic cleft (Figure 19-5). Receptors on the adjoining neuron bind with the neurotransmitter causing an alteration in the receptor’s configuration. These neurotransmitter-gated ion channels act as an internal pore allowing Na+ to flow down their electrochemical gradient into the cell. As the intracellular concentration of Na+ rises, the charge within the cell becomes positive causing a depolarization of the membrane. The result is a propagating electrical signal known as an action potential. This process repeats until the target tissue has been reached.
Figure 19-5.
The Synaptic Cleft. After the nerve impulse reaches and depolarizes the presynaptic membrane, the influx of Ca2+ results in the fusion of presynaptic vesicles to the surface of the membrane. The neurotransmitter released into the cleft binds to the receptor site on the postsynaptic membrane, which will propagate the action potential onto the adjacent neuron. [Adapted with permission from Kibble JD and Halsey CR: The Big Picture: Medical Physiology, 1st edition, McGraw-Hill, 2009.]
Alzheimer’s Disease (AD): AD is a progressive and fatal degenerative disease of the brain and the leading cause of dementia. Alzheimer’s is due to the accumulation of amyloid plaques between neurons and neurofibrillary tangles (NFTs) within the neurons. The exact mechanism of how the plaques and tangles affect brain function is not fully understood. Patients vary in both amount and ratio of plaques versus tangles. However, patients who have primarily only amyloid plaques deteriorate slower than those with primarily tangles. Amyloid plaques consist of β-amyloid protein, which is a protein fragment of amyloid precursor protein (APP). Normally, these protein fragments are degraded and eliminated via one of two pathways. In the case of AD, APP is cleaved by β-secretase and γ-secretase to form amyloid-β-derived diffusible ligands. The accumulation of the amyloid forms hard, dense plaques. These plaques physically alter the architecture of the surrounding tissue, and therefore alter normal neural functioning. In addition, amyloid deposits cause mitochondrial dysfunction leading to premature, scheduled cell death (apoptosis). The second major finding in AD is NFT. These tangles are made of six isoforms of tau proteins, highly soluble microtubule-associated proteins found normally in healthy brains. In a healthy person, phosphorylation of tau protein destabilizes axonal microtubules, allowing normal neuron function. In AD, the process is altered when hyperphosphorylation of tau results in the formation of NFTs. All six tau isoforms are present in the hyper-phosphorylated state. As they are altered, they become insoluble aggregates within the neuron leading to instability in the neural tubes. This impacts normal nerve conduction within the brain. |
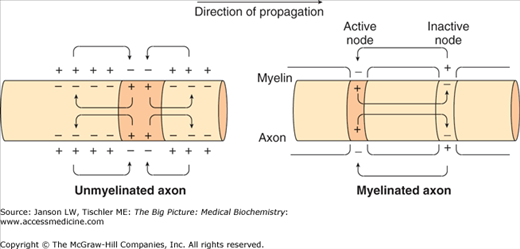
Myelin sheaths enable action potentials to travel faster than in unmyelinated axons and at decreased energy expenditure (Figure 19-6). The unsheathed nodes of Ranvier contain a high density of voltage-gated ion channels. The action potential is conducted along the axon via conduction jumping from one node of Ranvier to the next.
Figure 19-6.
Effect of the Myelin Sheath on Nerve Impulse Propagation. Positive charges from the membrane ahead of and behind the action potential flow in the area of negative charge as the potential travels down the axon (see direction of propagation). Unmyelinated nerves propagate the nerve impulse by slow, continuous conduction. Nerves with a myelin sheath are able to conduct the nerve impulse much quicker via saltatory conduction from one node of Ranvier to the next. [Adapted with permission from Barrett KE, et al.: Ganong’s Review of Medical Physiology, 23rd edition, McGraw-Hill, 2010.]
Repolarization is dependent upon several factors. The sodium channels are inactivated and cannot reopen until membrane polarization is reset to the resting membrane potential of -70mV. Inactivity of the sodium channels causes a consequent drop in sodium permeability and an increase in potassium conductance. During this time, the membrane is unable to repeat excitation (refractory period). Eventually, the sodium–potassium pump re-establishes the original baseline gradients and the cell is returned to its resting potential.
Autonomic Nervous System
The PNS is divided into the somatic nervous system and ANS. The somatic nervous system includes voluntary movements of skeletal muscles as well as touch sensation, vision, and hearing. The ANS coordinates and maintains a steady state among internal organs, such as regulation of cardiac muscle, smooth muscle, and glands. It is considered to be an involuntary system. The ANS is further subdivided into the sympathetic and parasympathetic nervous systems, the activity of which is integrated by the hypothalamus. An autonomic nerve pathway from the CNS to a visceral effector consists of two major neurons that synapse in a ganglion outside of the CNS: the preganglionic and postganglionic neurons. This differs from the somatic nervous system, wherein a single motor neuron travels from the CNS to the innervated tissue.