Overview
The cardiovascular system, including the heart and blood vessels, is responsible for the circulation of blood to all tissues of the body while carrying away carbon dioxide and waste products. Cardiac muscle shares many attributes with skeletal and smooth muscles but represents a separate muscle group with distinct structure, function, and regulation. Key in this function is the self-activating nature of particular cardiac cells that normally provide an ordered contraction of the cardiac chambers. Blood vessels contain smooth muscle cells regulated by a variety of signal molecules and also play an important role in maintenance of blood pressure and oxygen/nutrient distribution. Diseases of these blood vessels, especially hypertension and atherosclerosis, cause much of the illness and most of the deaths in the developed world.
Cardiac Muscle Structure and Function
The heart is derived from the mesoderm and provides the force that propels oxygenated blood to all cells of the body and returns deoxygenated blood back to the lungs (Figure 16-1). The heart comprises specialized striated muscle termed cardiac muscle.
Figure 16-1.
Sectional View of Heart. Basic anatomy of the heart is indicated, including chambers, major blood vessels, and conducting system (yellow), including the sinoatrial node, atrioventricular node, and Purkinje fibers. [Reproduced with permission from Barrett KE, et al.: Ganong’s Review of Medical Physiology, 23rd edition, McGraw-Hill, 2010.]
Increased numbers of mitochondria allow continuous high-level production of adenosine triphosphate (ATP) to support continuous function without fatigue. During periods of low oxygen, anaerobic respiration can provide enough energy for contraction to continue.
Utilizes lipids [fatty acids and triglycerides (60%)] as the major energy source followed by carbohydrates (35%), amino acids, and ketone bodies (5%).
Contains fewer but larger T tubules and intercalated discs that allow the rapid and synchronous spread of action potentials between adjacent cells, allowing the coordinated contraction of cardiac muscle.
Relies on the rapid release and re-uptake of intracellular calcium stores to convert the electrical signal of the action potential into the mechanical work of contraction.
Because cardiac cells and smooth muscle cells rely strongly on an L-type (“longlasting”) calcium channel for contraction, the medication class of calcium channel blockers (CCBs) are used to treat high blood pressure and certain cardiac disorders. In the heart, CCBs decrease the total calcium released and both the heart rate and force of the contraction, thereby reducing oxygen demand. This effect of CCBs is particularly useful in helping to control abnormal heart rhythms such as atrial fibrillation. In blood vessels, the decreased calcium-induced contraction results in dilation of the blood vessels (by decreasing the ability of the smooth muscle to contract) and lowers the resistance of those vessels. Additional cardiovascular uses of CCBs include reducing the outflow gradient in hypertrophic cardiomyopathy and reducing pulmonary arterial resistance in pulmonary hypertension. CCBs are also used in the treatment of epilepsy via their effect on neurons.
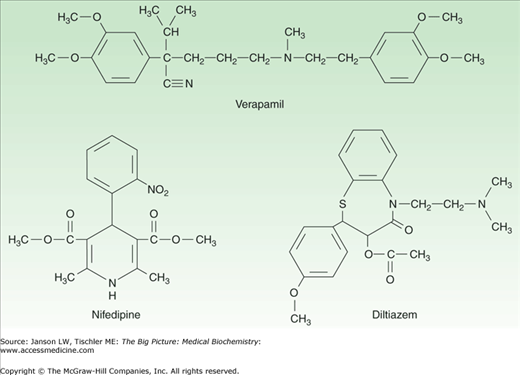
CCBs are often separated into dihydropyridines, most commonly used for hypertension because of greater affinity for receptors in vascular smooth muscle, and non-dihydropyridines, which are relatively cardiac selective and less likely to cause reflex tachycardia. Benzothiazipines such as diltiazem have intermediate characteristics. These three classes are summarized in Table 16-1 and Figure 16-3.
Class | Examples | Primary Effect(s) | Medical Use |
|---|---|---|---|
Dihydropyridine | Amlodipine, felodipine, and nifedipine | Dilation of blood vessels, smooth muscle dilation, and decreased vascular resistance. | Predominately used for blood pressure control. Less favored for angina because of reflex tachycardia. |
Phenylalkylamine | Verapamil | Decreased rate and force of cardiac contraction; also effective for coronary artery spasm (Prinzmetal’s angina). | Blood pressure control in patients suffering with angina, Prinzmetal’s angina, rate control in atrial fibrillation. |
Benzothiazepine | Diltiazem | Decreased rate and force of cardiac contraction and dilation of blood vessels, decreasing vascular resistance. | Blood pressure control in patients with or without angina. |
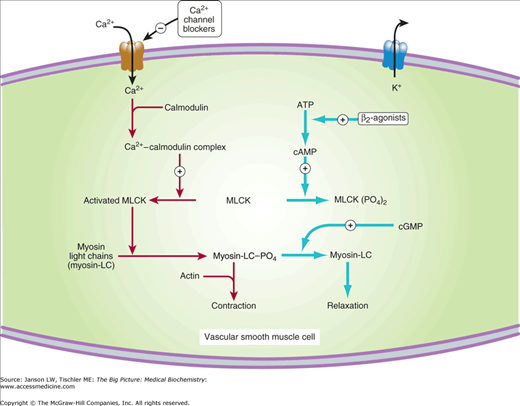
Figure 16-2.
Mechanism of Calcium Activation of Smooth Muscle Cells. Calcium ions (Ca2+) activate intermediary signaling molecules such as calmodulin and myosin light-chain kinase (MLCK) to phosphorylated regulatory myosin light chains, leading to contraction of vascular smooth muscle cell. Calcium channel blockers inhibit the influx of calcium, leading to inhibition of this process and resulting vascular smooth muscle relaxation. One effect of β-agonists/blockers leads to relaxation of vascular smooth muscle as shown. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate. [Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009.]
Figure 16-3.
Representatives of Calcium Channel Blocker Classes. Calcium channel blocker medications can be divided into three basic classes depending on their mechanism, including phenylalkylamines (e.g., verapamil), dihydropyridines (e.g., nifedipine), and benzothiazepines (e.g., diltiazem). See text and Table 16-1 for further discussion. [Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009.]
Sinoatrial and Atrioventricular Nodes
Particular cardiac cells located in the sinoatrial (SA) node (Figure 16-1) have the ability to initiate an action potential (Chapter 12), which then spreads through the muscle cells in the upper part of the heart (right and left atria) via the intercalated disks, resulting in a contraction of these two chambers. In normal sinus rhythm, the SA node initiates action potentials at 60–100/min that are conducted to the atrioventricular (AV) node, which coordinates the sequential contraction of the upper (atrial) and then lower (ventricular) chambers of the heart. If the SA node fails to provide action potentials, the AV node can independently generates action potentials at 40–60 beats per minute. Subsequent transmission of the action potential to the ventricles is via specialized heart cells (cardiomyocytes) in the right and left bundle branches and Purkinje fibers that spread throughout the ventricles. These conduction cells contain increased numbers of sodium ion channels and mitochondria while containing fewer cardiac muscle fibers. If both the SA and the AV nodes fail to provide an action potential, the Purkinje fibers or ventricular myocytes can also produce a coordinated contraction at rates slower than the AV node.
Although the SA and the AV nodes and Purkinje fibers can independently initiate and continue action potentials, which provide heart contractions, the vagal nerves (parasympathetic nerves causing a decreased SA node rate and force of contraction) and thoracic nerves T1–4 (sympathetic nerves resulting in increased SA node rate as well as force of contraction) also convey neurological influences on heart rate. Hormones/neurotransmitters such as epinephrine (adrenaline) and dopamine increase heart rate and/or the amount of blood pumped per contraction (stroke volume). Epinephrine works via binding to α1-, α2-, β1 and β2-adrenergic, G-protein-coupled receptors. The receptor signal transduction includes activation of phospholipase C to increase production of inositol phosphates/diacylglycerol, protein kinase C activity, and the release of Ca2+ (α1-/Gq protein). Additionally, there will be inhibition (α2-/Gi protein) or activation (β1 and β2-/Gs protein) of adenylyl cyclase to decrease or increase cyclic adenosine monophosphate (cAMP) production and protein kinase A activity, respectively (see Chapter 8 for review of G-protein activities).
Dopamine: Dopamine is a neurotransmitter that not only affects the central nervous system but also elicits dose-dependent effects on blood vessels and cardiac muscle via Dopamine (D) receptors and α1– and β1-adrenergic receptors. It is frequently used in the intensive care unit to support blood pressure. At low doses (2–5 μg/kg/min), binding is primarily to D1 and D5 receptors found on blood vessels, especially in the kidneys, activating a Gs-receptor (adenyl cyclase/cAMP), resulting in increased urine production. Dopamine when delivered at a rate of 5–10 μg/kg/min binds to β1-adrenergic receptors in cardiac muscle, producing increased contraction rate and force for treatment of shock/heart failure patients. Dopamine provided at 10–20 μg/kg/min binds to α1-adrenergic receptors, resulting in contraction of blood vessels and increased resistance/blood pressure.
Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009. |
The Cardiac Cycle
The Cardiac cycle is an autonomous process that results in the organized, sequential, contraction of the chambers of the heart, temporally coordinated to produce efficient pumping throughout the body of blood, which then returns to the heart and lungs for re-oxygenation. The cardiac cycle includes steps that result in heart sounds, which produce the characteristic electrical signals seen on an electrocardiogram (ECG) tracing, including the P-wave (atrial depolarization), QRS-complex (ventricular depolarization), and T-wave (ventricular repolarization).
The initiation of cardiac muscle contraction relies on voltage-dependent calcium channels (VDCCs). The predominant VDCC on cardiac cells is the L-type channel, which is composed of an α1 subunit with six transmembrane α-helices that form a membrane channel or pore (Chapter 8), but which also responds to voltage changes by alterations in conformation that blocks or allows Ca2+ passage. Along with the α1 pore, this channel includes other subunits. The disulfide-linked (Chapter 1) α2δ subunits anchor the α1 protein in the membrane and augment its response to voltage changes. A β subunit both enhances α1 expression on the membrane and contains guanylate kinase activity that catalyzes the reaction ATP + GMP ↔ ADP + GDP. The conversion of GMP to GDP serves as a regulatory mechanism to augment the voltage sensitivity for the α1-pore (i.e., β-subunit activity allows smaller depolarizations to result in channel opening). Finally, a γ-subunit is believed to be of little significance in cardiac muscle, although it may play an important regulatory role in skeletal muscle contraction. Sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) also plays a prominent role in cardiac cell contraction (see Chapter 12).
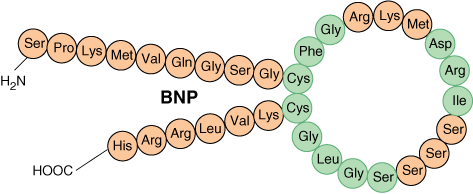
B-type Natriuretic Peptide (BNP): BNP is a 32-amino acid polypeptide (Chapter 18) that is secreted by the ventricles when cardiac cells are excessively stretched. BNP binds to atrial natriuretic peptide receptors, activating a guanylyl cyclase producing cyclic GMP (cGMP), and, via a cGMP-dependent protein kinase, producing relaxation of blood vessels. Rises in BNP levels are seen in congestive heart failure (CHF) where it reduces the systemic resistance and blood volume, thereby reducing the work of the ailing heart. Measurement of BNP is useful to both assess and follow patients with CHF.
Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009. |
β-Blockers: β-Blockers (all ending in “-lol”) represent a class of medications used for the treatment of high blood pressure, irregular heart beat, chest pain (angina), and heart attack therapy. β-Blockers inhibit β1– and β2-adrenergic receptors in the heart and blood vessels. Stimulation of β1-receptors on cardiac cells by molecules such as epinephrine normally leads to increased heart rate and augmented contraction that increases myocardial oxygen requirements. Blockade of these receptors reduces oxygen demand and can also help the heart retain a normal geometry as it heals. Stimulation of β2-receptors on vascular smooth muscle relaxes (dilates) blood vessels (see Figure 16-2). So, vasoconstriction, for example, resulting from certain drugs of abuse, may be exacerbated by β-blockers.
Reproduced with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009. Like CCBs, β-blockers also have “classes” related to their relative effect(s) on cardiac muscle or vascular smooth muscle. Relatively specific β1-blockers such as metoprolol affect mainly cardiac muscle, decreasing heart rate and the force of contraction and reducing work of the heart and oxygen requirements. This effect is a key in the treatment of heart attack/angina victims. Nonspecific β-blockers, of which the archetype is propranolol, inhibit β2-receptors as well. β-blockers also interact with β-adrenergic receptors in other organs (e.g., eye, brain, kidney, and lung) and may decrease intraocular pressure (glaucoma) and may improve migraine headaches, anxiety/PTSD. They also reduce renin release which limits conversion of angiotensinogen to angiotensin 1. This reduces angiotensin II with subsequent reduction in aldosterone from kidneys (Chapter 18), reducing blood volume. Both effects lower blood pressure. Their effect on the lung may worsen bronchial constriction in asthma attacks. β-blockers are also used in many cardiac arrhythmias, CHF (via their relaxing effects on the heart but also their effect on renin/aldosterone), mitral valve prolapse, portal hypertension and any resultant bleeding of esophageal varices, pheochromocytoma, and hypertrophic cardiomyopathy. However, the cross-reactivity of some β-blockers for differing receptors and tissues and the ability of some β-blockers to both inhibit and stimulate the same receptors depending on concentration leads to a number of unwanted side effects that must be carefully understood, monitored, and managed by the clinician. |