Overview
The immune system, composed of antigens, antibodies, and several specialized leukocyte (white blood cell) types, offers the human body adaptable protection against infections and invasion by foreign molecules and cells. Besides responses by antibodies and cells, the immune system is also composed of a complement-activated pathway that allows an alternative to respond to infectious organisms.
Overview of the Immune System
The human immune system is responsible for generating a protective response to infective organisms (e.g., bacteria, viruses, and parasites) and foreign cells (e.g., tumor and transplant) while both recognizing the host body and limiting damage to itself (self–nonself theory). As attacks are always evolving, the immune system must be capable of adapting to each new challenge. The immune system also provides an inflammatory response to trauma. When tissue or cells are damaged, molecules that act as endogenous danger signals can be released. These danger signals may include molecules such as uric acid (produced by purine metabolism, Chapter 4), which, if present at high concentrations, can form crystals that innate immune sensors recognize and thus activate immune and inflammatory responses.
Innate immunity refers to preformed host defense systems that can provide immediate host defense activities without first being trained to distinguish self from invader. Elements of the innate immune system often contain structural recognition motifs that allow them to identify likely pathogens to target. Molecules that are found in microbes without structural homologs in human cells, such as flagellin or unmethylated deoxyribonucleic acid (DNA), are other examples of danger signals that induce innate immune activation when their structure is recognized by innate immune sensors.
The adaptive immune system is capable of generating a response very specific to the structure of an invading organism or molecule (antigen, see below) as well as the ability to remember that structure via memory cells (immunological memory). The repeat activation of the immune system by an antigen that has previously induced an immune response is normally much quicker and stronger. The invading pathogen serves as a target, which elicits a response specific for that antigen from immune cells. This response may include the production of a specific antibody against that pathogen (humoral immune system) or the activation of immune cells that either attack and kill the offending organism or orchestrate this activity, the cell-mediated immune system. These cell types include lymphocytes (including T and B lymphocyte types), monocytes, macrophages, dendritic cells (DCs), neutrophils, eosinophils, and basophils.
Passive immunity involves antibody molecules that are transferred to the baby from the mother’s active immune system through the placenta. Immunoglobulin (Ig) G (see below) is the single antibody type that binds to a neonatal Fc receptor and is endocytosed via pinocytosis. Newborns also receive IgA antibodies (see below) via breast milk, which provides initial protection against pathogenic microorganisms. Passive immunity is considered to be a short-term system, which is taken over in the first year or two of life by the adaptive immune system.
Immunodeficiencies: Medical conditions known as immunodeficiencies are characterized by the absence or malfunction of a component of the immune system, leading to the inability to fight infectious disease. More than 120 congenital immunodeficiencies have been recognized. External causes of immunodeficiency include poor diet, the effects of immunomodulating drugs, and infectious diseases including human immunodeficiency virus (HIV)/acquired immune deficiency syndrome. |
Antigen
Antigen, originally termed from the phrase “antibody generator,” is a molecule (normally protein or carbohydrate in source) that can stimulate the immune system to make antibodies and/or initiate a cell-mediated response. Self-antigens are present on all cells of the host organism. Self-reactive immune cells are typically deleted or maintained in states of impaired reactivity to prevent the development of autoimmunity. Many pathogenic microbes and cancer cells can use some of these same mechanisms to evade immune attack. Other “opportunistic” organisms may induce aggressive responses from intact immune systems (that effectively kill the organism) but can still lead to disease in immunocompromised hosts.
The specific structural surface recognized by cells of the adaptive immune system is known as an epitope. Several antibodies may recognize various parts of one antigen (various epitopes). To be recognized by T cells in the cell-mediated immune system, antigens must be presented on the cell surface of particular immune cells (see below).
Antibody
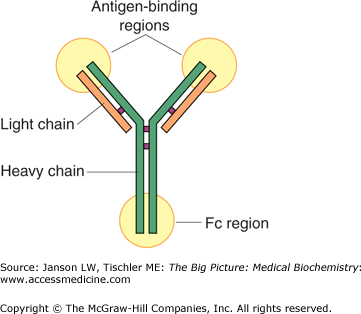
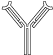
An antibody is a large, Y-shaped protein (Figure 15-1) responsible for immunological identification and binding of antigens and, ultimately, for protection against invading pathogens. Another name for an antibody is immunoglobulin, normally abbreviated as “Ig.”
Figure 15-1.
Basic Structure of an Antibody Molecule. The antibody molecule is a Y-shaped, tetramer protein, composed of two heavy chains and two light chains, held together by hydrophilic/hydrophobic forces and disulfide bonds. The body of “Y” is composed of a constant protein structure and a highly variable end, which defines the affinity of a particular antibody for one antigen epitope. The Fc region of the molecule may bind to the surface of receptors of several cell types. [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
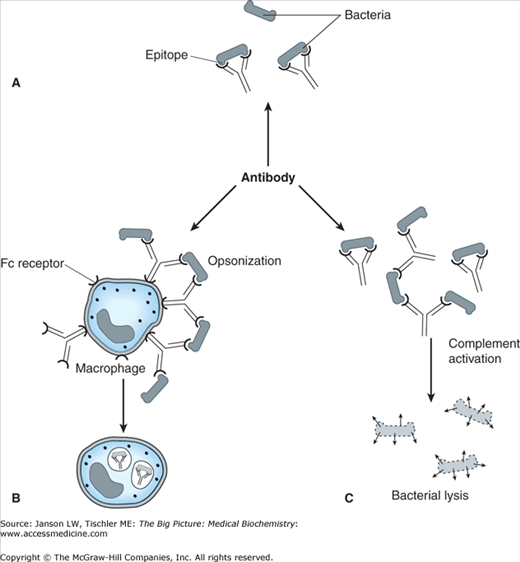
Figure 15-2.
Summary of Antibacterial Antibody Functions. Antibodies can elicit a number of reactions resulting in the elimination of bacteria, including (A) recognition and binding to bacteria, which allows recognition by other immune system components to include (B) opsonization, leading to recognition and phagoyctysis by macrophages, and (C) binding leading to complement activation (see below), leading to bacterial lysis. [Adapted with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009.]
Antibodies exist in five types: IgA, IgD, IgE, IgG, and IgM. The basic structure, source, and function of each are summarized in Table 15-1.
Antibody | Structure | Source | Function |
|---|---|---|---|
Immunoglobulin (Ig) A |
Contains a J chain subunit required for secretion. | B lymphocytes in the mucosal lining of tear and saliva ducts, lungs, intestines, genito-urinary tract, and prostate. Also secreted in breast milk and tears. | Provides initial immunological protection against invading organisms found in these sites. Breast milk IgA provides important immunological protection for newborns. |
IgD |
| Produced by B lymphocytes (plasma cells). Usually coexpressed on plasma membrane with IgM. Can be secreted. | Regulation of B lymphocyte activation. May also activate basophils and mast cells. |
IgE |
| Produced by B lymphocytes (plasma cells). | Involved in type 1 hypersensitivity “allergic” reactions and response to parasitic worms and protozoan. |
IgG |
| Produced by B lymphocytes (plasma cells). Most abundant (∼75%) of all Igs in human body. | Major Ig of secondary immune response to viral, bacterial, fungal, and other pathogen invasions. Involved in types II and III hypersensitivity reactions and helps induce phagocytosis. Only Ig crossing placenta for passive immunity. |
IgM |
Contains a J chain subunit required for secretion. | Produced by B lymphocytes (plasma cells). Because of its large size, most of it is found in the blood serum. First Ig produced by fetus. | Involved in initial response to new antigen and, thus, serves as a marker for recent infection. Also activates complement. Main antibody for blood type determination and blood transfusion incompatibility. |
Cells Associated with the Immune System
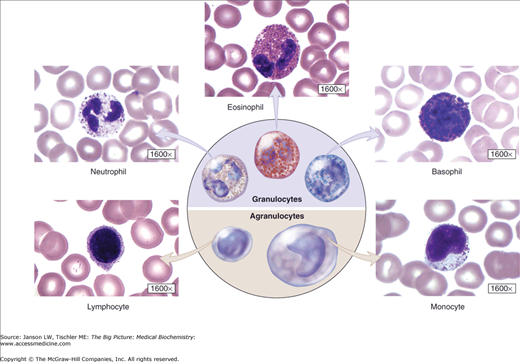
Cells of the immune system, collectively called leukocytes or white blood cells, can be categorized as lymphocytes, monocytes, neutrophils, eosinophils, and basophils (Figure 15-3).
Figure 15-3.
White Blood Cells. Overview of white blood cells, including those derived from granulocytes and agranulocytes. A laboratory full blood count usually includes total white blood cells and a differential measurement of the five major types of white blood cells. Normal values are as follows: neutrophils (45%–60% of total), lymphocytes (25%–35% of total), monocytes (3%–7% of total), eosinophils (1%–3% of total), and basophils (<1% of total). See Appendix II for further details. [Reproduced with permission from Mescher AL: Junqueira’s Basic Histology Text and Atlas, 12th edition, McGraw-Hill, 2010.]
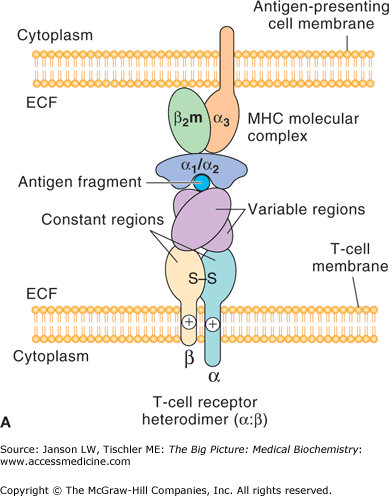
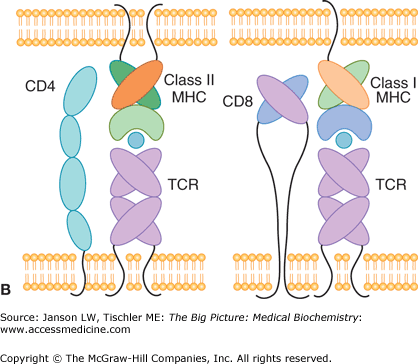
T lymphocytes contain a T-cell receptor (TCR) and CD4 or CD8 coreceptor on their plasma membrane (Figure 15-4A–B). Each TCR recognizes a molecule’s distinct epitope structure and allows the specificity needed for a controlled immune reaction (Figure 15-4A). T lymphocytes with a CD8 coreceptor recognize antigen presented by Class I major histocompatibility complex (MHC) molecules on the surface of a wide variety of cells, and frequently are effectors of direct cell killing (cytotoxic T cells). T lymphocytes with a CD4 coreceptor (helper T cells or Th cells) recognize antigen presented by Class II MHC molecules on the surface of specialized antigen-presenting cells (APCs, such as macrophages, DCs, and some B cells), and frequently orchestrate the responses of other immune cells. This process of antigen presentation is summarized in Figure 15-4B.
Figure 15-4.
A. T-Cell Receptor Recognition of Antigen. Basic structure of the heterodimeric T-cell receptor (bottom), showing the α- and β-subunits and the constant and variable region that recognize the epitope of the antigen fragment presented by an antigen-presenting cell (top). ECF, extracellular fluid; MHC, major histocompatibility complex. B. Binding of T Cells to MHC Complex. CD4 (helper T) cells have a T-cell receptor (TCR) and CD4 coreceptor that recognize antigen (blue circle) bound to MHC Class II receptor on an antigen-presenting cell (left panel). CD8 (cytotoxic T) cells bind via their TCR to antigen (blue circle) presented by MHC Class I molecules (right panel). MHC, major histocompatibility complex; TCR, T-cell receptor. [Reproduced with permission from Barrett KE, et al.: Ganong’s Review of Medical Physiology, 23rd edition, McGraw-Hill, 2010.]
Recognition of a specific antigen in the antigen–MHC complex by the TCR of the T lymphocyte cells (Figure 15-5, left) activates a signaling process, involving a peripheral membrane leukocyte-specific tyrosine kinase (Lck), which is tethered to the inside of the plasma membrane by lipid residues linked to its amino-terminal end. Lck also contains two highly conserved amino acid sequences, known as sarcoma homology domains, and binds to the cytoplasmic tails of either the CD4 or CD8 coreceptor molecules. Upon activation, Lck phosphorylates a subunit of the TCR at a highly conserved tyrosine separated from a leucine or isoleucine by two other amino acids. A double repeat of this four amino acids sequence, the two separated by 7–12 amino acids, is known as an immunoreceptor tyrosine-based activation motif (ITAM) and is found on subunits of the TCRs as well as B-cell receptors (see below). Phosphorylation of the two tyrosines of the ITAM allows binding of other signaling proteins resulting in subsequent phosphorylation of another tyrosine kinase called zeta-chain-associated protein kinase-70 (ZAP-70). ZAP-70 activation eventually leads to phosphorylation and activation of phospholipase C-γ 1; secretion of calcium from the endoplasmic reticulum; and altered gene expression by the actions of nuclear factor of activated T cells, nuclear factor kappa B (NF-κB), and activator protein 1 (AP-1) transcription factors, among others, in the cell nucleus. Cytotoxic T cells produce perforin and granulysin proteins, which create open channels in the plasma membrane of infected cells, and granzymes, serine protease enzymes, both of which lead to cell death. Cytokines including interleukin-2 (IL-2) are also expressed, leading to sustained replication and activity of the lymphocytes.
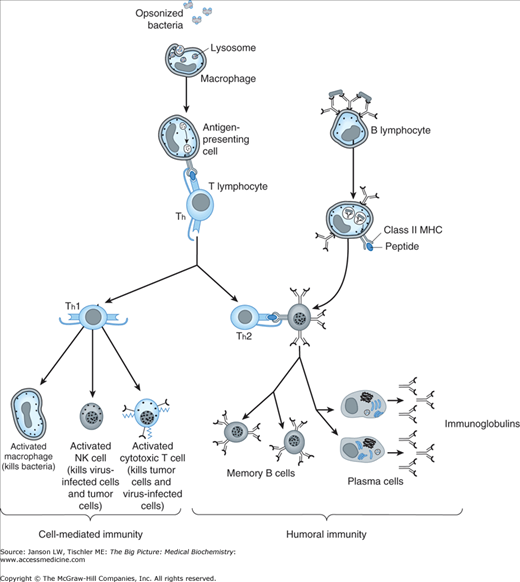
Figure 15-5.
Comparison of Cell-Mediated and Humoral Immunity. Cell-mediated immunity (left) involves the presentation of antigen derived from ingested foreign molecules (e.g., bacteria) for T helper (Th) lymphocyte recognition and Th1 activation of cells such as macrophages, natural killer, and cytotoxic T cells (see the text). Humoral immunity (right) results from recognition of the foreign molecule by an antibody, which is already expressed on a B lymphocyte followed by interaction with a Th2 cell and differentiation into memory B and plasma cells, the latter of which express large amounts of antibody against the offending molecule. MHC, major histocompatibility complex; NK, natural killer. [Adapted with permission from Katzung BG, et al.: Basic and Clinical Pharmacology, 11th edition, McGraw-Hill, 2009.]
As the name suggests, helper T lymphocytes assist a number of other immune cells including subsequent activation of B lymphocyte antibody production (see below), macrophage phagocytosis (see below), and activation of cytotoxic T cells. Th cells are a major producer of cytokines. Th cells known as Th1 cells produce the cytokines interleukin-12 and interferon-γ (IFN-γ) via Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signaling (Chapter 8), leading to both differentiation and production of more Th1 cells. Additionally, Th1 cells cause (a) increased expression of MHC Class I molecules on normal cells, (b) production of adhesion molecules that promote white blood cell migration to sites of infection, (c) decreased production of Th2 cells (see below), (d) increased lysosomal activity and antigen presentation by macrophages, (e) increased natural killer (NK) cell activity (see below), and (f) increased production of nitric oxide, which can assist in bacterial and viral killing. In sum, these signals activate multiple cells to fight infecting organisms while downregulating other T-cell pathways. One alternative T-cell set known as Th2 cells
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree