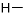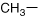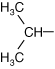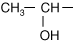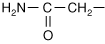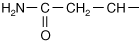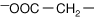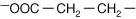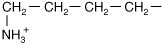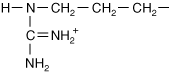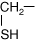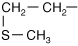Overview
Amino acids are the basic building blocks of proteins and serve as biological molecules in their own right with a variety of functions. Amino acids are often categorized as essential or non-essential, depending on the ability of the body to manufacture each amino acid versus requirement for ingestion in the diet. Although several hundred amino acids exist, 20 play the predominant role in the human body. Each amino acid has a characteristic R-group that determines its chemical nature and, therefore, how it will interact with other amino acids, other molecules, and with its environment.
Amino acids link together via peptide bonds to form peptides and proteins. These peptides and proteins fold into their final three-dimensional shape as the result of hydrophobic, hydrophilic, hydrogen bonding, and ionic bonding forces (among others) that result from the amino acids in the peptide chain, including the characteristics of their R-groups. Proteins may be categorized as enzymes, structural proteins, motor proteins, and transport/channel proteins. The specific roles of amino acids and proteins in the synthesis of other molecules and in the functions of organ systems and the human bodies will be explored in detail in subsequent chapters.
Amino Acids—Structure and Functional Groups
Amino acids are the basic building blocks of proteins. Twenty amino acids make up proteins in living organisms; several hundred more amino acids perform specialized functions in human and non-human biology. Amino acids are often described as
- Essential (must be obtained directly from food)
- Non-essential (the human body is able to produce them on its own).
There is some debate about the exact definitions of these terms, but 8–10 amino acids are usually deemed essential and 10–12 are non-essential (see Table 1-1).
Name | R-Group | Notes | |
|---|---|---|---|
Hydrophobic | Glycine |
| Smallest R-group |
Alanine |
| Methyl R-group that normally folds easily within a protein | |
Valine |
| Bulky structure can impact folding of protein | |
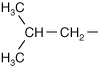
Leucine |
| Bulky structure can impact folding of protein | |
Isoleucine |
| Bulky structure can impact folding of protein | |
Phenylalanine |
| Aromatic ring | |
Tryptophan |
| Indole ring | |
Hydrophilic | Serine |
| Hydroxyl (OH) group with partial negative (−) charge (not shown); may be phosphorylated |
Threonine |
| Hydroxyl (OH) group with partial negative (−) charge; may be phosphorylated | |
Asparagine |
| Amino (NH2) group with partial positive (+) charge (not shown) | |
Glutamine |
| Amino (NH2) group with partial positive (+) charge (not shown) | |
Charged | Tyrosine |
| Aromatic ring with hydroxyl group, giving partial or full negative (−) charge (not shown); may be phosphorylated |
Aspartic acid |
| Negative (−) charge from COO− | |
Glutamic acid |
| Negative (−) charge from COO− | |
Lysine |
| Positive (+) charge from NH3+ | |
Arginine |
| Positive (+) charge from NH2+ | |
| Special | Proline |
| β-turns |
Cysteine |
| Disulfide bonds | |
Methionine |
| Sulfur atom | |
Histidine |
| Partial or full positive (+) charge from NH+ (not shown) |
Succotash, a combination of corn and lima beans, was used by Native American hunters and warriors. Light in weight, easy to carry, and simple to prepare, succotash contains all the essential required amino acids needed by humans and kept these travelers well nourished and healthy during long trips away from their settlements.
|
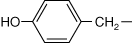
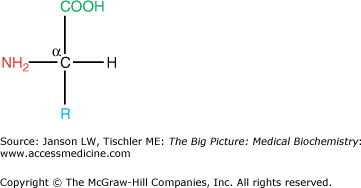
Every amino acid has four components linked together with a central carbon atom (Figure 1-1):
Amino group
Carboxylic acid group
Hydrogen atom
R-group, which varies with each amino acid
As amino acids are identical except for their R-group, four R-group characteristics classify the amino acids (see also Table 1-1).
Hydrophobic (Water Hating) R-groups: These amino acids prefer to be inside a folded protein or covered by another part of a protein or a lipid membrane (Chapter 8) where they are hidden from the external water environment.
Hydrophilic (Water Loving) R-groups: With partial charges from the hydroxyl (OH−) or amino (NH3+) parts of these R-groups, these amino acids prefer to be at or near the surface of a protein where they interact with surrounding water molecules. An exception would be the surface portion of a membrane protein that interacts with the hydrophobic region of the phospholipid bilayer (Chapter 8). Hydrophilic R-groups are often important at the active site of an enzyme (Chapter 5).
Charged R-groups: Either positively or negatively charged, these amino acids prefer to be at the surface of a folded protein or in contact with other charged atoms/molecules.
Special R-groups: There are four amino acids with special quality R-groups.
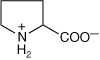
- Proline has a glutamate R-group that has bonded onto itself (see Figure 1-2A) forming an “imino” acid. Proline is often found at sharp turns of folded proteins (Figure 1-2B).
- Cysteine has a sulfhydryl group that can bond with another cysteine sulfhydryl group to form a cystine “disulfide” bond (Figure 1-3). This bond can be either within one protein or between two different proteins and is important in making strong structures such as the protein keratin found in fingernails.
- Methionine has a sulfur atom contained within its R-group. Although it does not make disulfide bonds, this sulfur is seen at the site of some enzyme reactions or at special areas of protein structure.
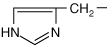
- Histidine has a unique imidazole ring containing two nitrogen atoms, which can be uncharged or positively charged. This unique characteristic makes the histidine R-group important in enzyme reactions that make or break bonds.
- Proline has a glutamate R-group that has bonded onto itself (see Figure 1-2A) forming an “imino” acid. Proline is often found at sharp turns of folded proteins (Figure 1-2B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree