CHAPTER 20 Cerebellum
The cerebellum occupies the posterior cranial fossa, separated from the occipital lobes of the cerebral hemispheres by the tentorium cerebelli. It is the largest part of the hindbrain; in adults the weight ratio of cerebellum to cerebrum is approximately 1 : 10 and in infants 1 : 20. The cerebellum lies dorsal to the pons and medulla, from which it is separated by the fourth ventricle. It is joined to the brain stem by three bilaterally paired cerebellar peduncles, and these contain all the afferent and efferent fibres associated with the cerebellum.
EXTERNAL FEATURES AND RELATIONS
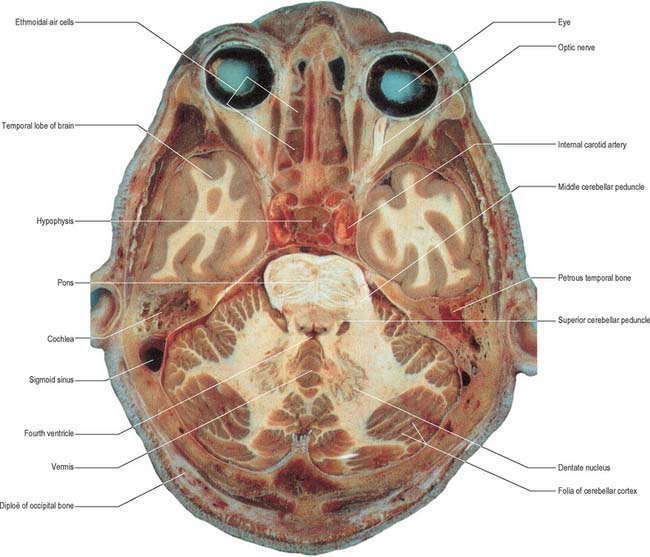
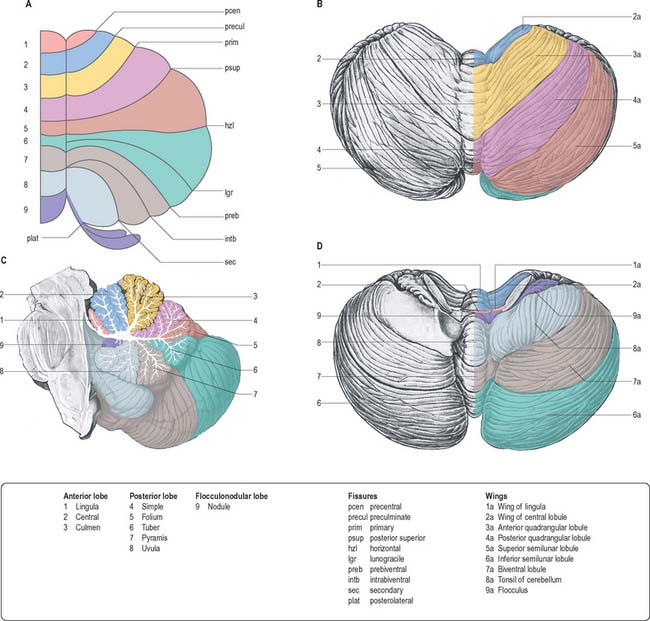
The cerebellum consists of two large, laterally located hemispheres which are united by a midline vermis (Figs 20.1, 20.2, 20.3). The superior surface of the cerebellum, which would constitute the anterior part of the unrolled cerebellar cortex, has a flat profile (Fig. 20.3). The paramedian sulci are shallow and the borders between vermis and hemispheres are indicated by kinks in the transverse fissures. The superior surface adjoins the tentorium cerebelli and projects beyond its free edge. The transverse sinus borders the cerebellum at the point where the superior and inferior surfaces meet. The inferior surface is characterized by a massive enlargement of the cerebellar hemispheres, which extend medially to overlie some of the vermis. Deep paramedian sulci demarcate the vermis from the hemispheres. Posteriorly the hemispheres are separated by a deep vallecula, which contains the dural falx cerebelli. The inferior cerebellar surface lies against the occipital squama. The shape of the surface facing the brain stem is irregular and forms the roof of the fourth ventricle and the lateral recesses on each side of it, while the cerebellar peduncles define the diamond shape of the ventricle when viewed from behind. Anterolaterally the cerebellum lies against the posterior surface of the petrous part of the temporal bone.
FUNCTIONAL DIVISIONS OF THE CEREBELLUM
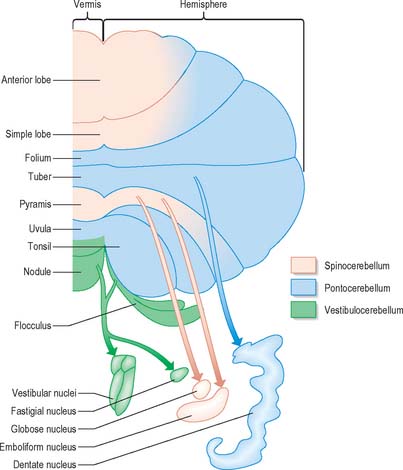
Functionally speaking, the cerebellum can be divided into a body, with inputs mainly from the spinal cord and pontine nuclei, and a flocculonodular lobe, which has strong afferent and efferent connections with the vestibular nuclei (Fig. 20.4). The body is subdivided into a series of regions dominated by their spinal or pontine inputs. The anterior lobe, simple lobule, pyramis and biventral lobules are the main recipients of spinal and trigeminal cerebellar afferents. Pontocerebellar input dominates in the folium, tuber and uvula, and throughout the entire hemisphere, including those regions that receive afferents from the spinal cord.
INTERNAL STRUCTURE
CEREBELLAR NUCLEI
Laterally, on either side within the white matter core, are four cerebellar nuclei. These are the dentate, emboliform, globose and fastigial nuclei. The dentate nucleus, which is located most laterally and is by far the largest, is the only nucleus easily visible to the naked eye (Fig. 20.1). It has the form of an irregularly folded sheet of neuronal cell bodies, with a medially directed hilum through which pass a mass of fibres mainly derived from dentate neurones and which form the bulk of the superior cerebellar peduncle. The emboliform and globose nuclei lie medial to the dentate and are equated to the nucleus interpositus (interposed nucleus) in lower species; the emboliform and globose nuclei may sometimes be referred to as the anterior and posterior interposed nuclei, respectively. Their efferent fibres join the superior cerebellar peduncle. The fastigial nucleus lies next to the midline, bordering on the roof of the fourth ventricle. A large proportion of the efferent fibres that leave this nucleus decussate within the cerebellar white matter and subsequently constitute the uncinate fasciculus, which passes dorsal to the superior cerebellar peduncle to enter the contralateral vestibular nuclei. Uncrossed fastigiobulbar fibres enter the vestibular nuclei by passing along the lateral angle of the fourth ventricle, and some fibres of the fastigial nucleus ascend in the superior cerebellar peduncle.
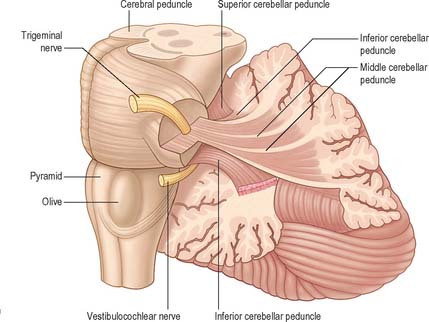
CEREBELLAR PEDUNCLES
Three pairs of peduncles connect the cerebellum with the brain stem (see Ch. 19; Fig. 20.5).
The superior cerebellar peduncle contains all of the efferent fibres from the dentate, emboliform and globose nuclei and a small fascicle from the fastigial nucleus. Its fibres decussate in the caudal mesencephalon, on their way to synapse in the contralateral red nucleus and thalamus. The anterior spinocerebellar tract reaches the upper part of the pontine tegmentum before looping down within this peduncle to join the spinocerebellar fibres entering through the restiform body.
CEREBELLAR CORTEX
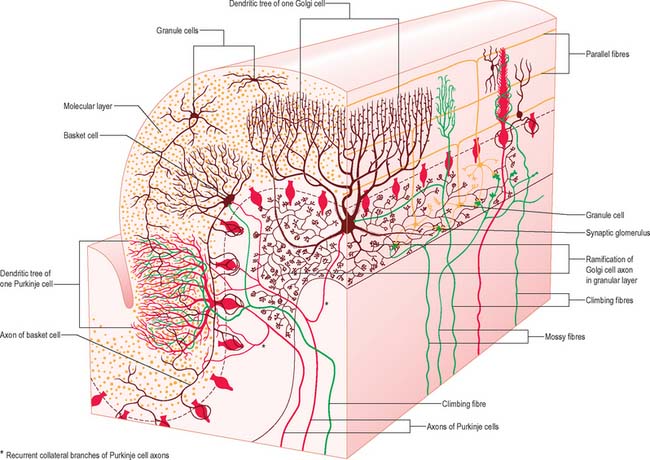
There are three main layers: molecular, Purkinje cell and granular (Fig. 20.6). The main circuit of the cerebellum involves granule cells, Purkinje cells and neurones in the cerebellar nuclei. Granule cells receive the terminals of the mossy fibre afferents (i.e. all afferent systems except the olivocerebellar fibres). The axons of the granule cells ascend to the molecular layer, where they bifurcate into parallel fibres, which are so called because they are oriented parallel to the transverse fissures and perpendicular to the dendritic trees of the Purkinje cells on which they terminate. Purkinje neurones are large and are the sole output cells of the cerebellar cortex. Their axons terminate in the cerebellar nuclei and in the vestibular nuclei. In addition to the dense array of parallel fibres, the dendritic trees of Purkinje cells receive terminals from climbing fibres which originate from neurones in the inferior olivary nucleus. The cerebellar cortex thus receives two distinct types of input: olivocerebellar climbing fibres, which synapse directly on Purkinje neurones, and mossy fibres, which connect to the Purkinje cells via granular neurones, whose axons are the parallel fibres.
The molecular layer is approximately 300–400 μm thick. It contains a sparse population of neurones, dendritic arborizations, non-myelinated axons and radial fibres of neuroglial cells. Purkinje cell dendritic trees extend towards the surface and spread out in a plane perpendicular to the long axis of the cerebellar folia. Purkinje cell dendrites are flattened. The lateral extent of the Purkinje cell dendrites is some 30 times greater in the transverse plane than it is in a plane parallel to the cerebellar folia. Parallel fibres are the axons of granule cells, the stems of which ascend into the molecular layer where they bifurcate at T-shaped branches. The two branches extend in opposite directions as parallel fibres along the axis of a folium. Parallel fibres terminate on the dendrites of the Purkinje cells and Golgi cells, which they pass on their way, and on the basket and stellate cells of the molecular layer. Dendritic trees of Golgi neurones reach towards the surface. Unlike the flattened dendritic tree of the Purkinje cell, Golgi cell dendrites span the territory of many Purkinje neurones longitudinally as well as transversely. These dendrites receive synapses from parallel fibres. Some Golgi cell dendrites enter the granular layer, where they contact mossy fibre terminals. The cell bodies of Golgi neurones lie below, in the superficial part of the granular layer. The molecular layer also contains the somata, dendrites and axons of stellate neurones (which are located superficially within the molecular layer) and of basket cells (whose somata lie deeper within the molecular layer). Climbing fibres, the terminals of olivocerebellar fibres, ascend through the granular layer to contact Purkinje dendrites in the molecular layer. Radiating branches from large epithelial (Bergmann) glial cells give off processes that surround all neuronal elements, except at the synapses. Their conical expansions join to form an external limiting membrane at the surface of the cerebellum.
The granular layer (Fig. 20.6) is about 100 μm thick in the fissures and 400–500 μm on foliar summits. There are approximately 2.7 million granular neurones per cubic millimetre. It has been estimated that the human cerebellum contains a total of 4.6 × 1010 granule cells, and that there are 3000 granule cells for each Purkinje cell.
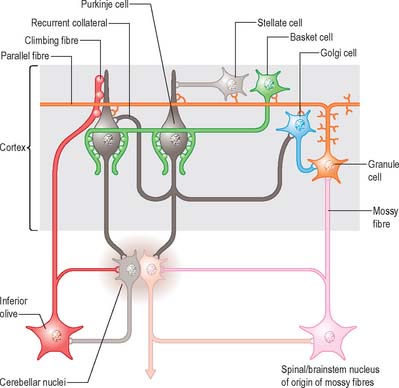
Of the five cell types to be described, the first four are inhibitory, liberating γ-aminobutyric acid (GABA), and the fifth is excitatory, liberating L-glutamate. Figure 20.7 summarizes their main connections.
Purkinje cells have a specific geometry, which is conserved in all vertebrate classes (Fig. 20.6). They are arranged in a single layer between the molecular and granular layers. Individual Purkinje cells are separated by about 50 μm transversely and 50–100 μm longitudinally. Their somata measure approximately 50–70 μm vertically and 30–35 μm transversely. The subcellular structure of the Purkinje cell is similar to that of other neurones. One distinguishing feature is subsurface cisterns, often associated with mitochondria, which are present below the plasmalemma of somata and dendrites and may penetrate into the spines. The cisterns are intracellular calcium stores which are important links in the second messenger systems of the cell.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree