5 Central nervous system
Basic concepts
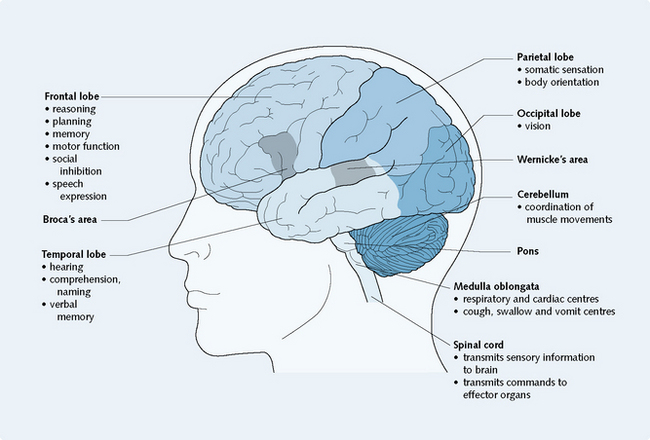
The central nervous system consists of the brain and the spinal cord, which are continuous with one another. The brain is composed of the cerebrum (which consists of the frontal, temporal, parietal and occipital lobes), the diencephalon (which includes the thalamus and hypothalamus), the brainstem (which consists of the midbrain, pons and medulla oblongata) and the cerebellum. The brain functions to interpret sensory information obtained about the internal and external environments and send messages to effector organs in response to a situation. Different parts of the brain are associated with specific functions (Fig. 5.1). However, the brain is a complex organ and is not yet completely understood.
Parkinson’s disease and parkinsonism
Parkinsonism is most commonly caused by Parkinson’s disease, though other causes exist.
Pathogenesis
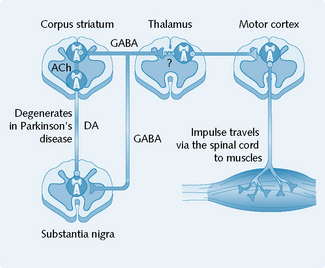
The main pathology in Parkinson’s disease is a progressive degeneration of the dopaminergic neurons of the substantia nigra, which project via the nigrostriatal pathway to the corpus striatum (Fig. 5.2). The inhibitory dopaminergic activity of the nigrostriatal pathway is, therefore, considerably reduced (by 20–40%) in people with Parkinson’s disease.
Untreated Parkinson’s eventually results in dementia and death.
Treatment of parkinsonism
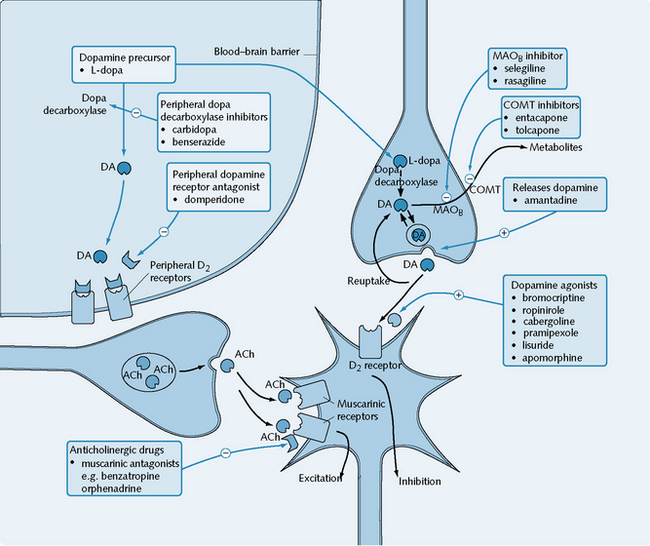
The treatment of parkinsonism is based on correcting the imbalance between the dopaminergic and cholinergic systems at the basal ganglia (Fig. 5.3). Two major groups of drugs are used: drugs that increase dopaminergic activity between the substantia nigra and the corpus striatum, and anticholinergic drugs that inhibit striatal cholinergic activity.
Drugs that increase dopaminergic activity
Dopamine precursors
An example of a dopamine precursor is levodopa (l-dopa).
Mechanism of action
l-dopa is the immediate precursor of dopamine and is able to penetrate the blood–brain barrier to replenish the dopamine content of the corpus striatum. l-dopa is decarboxylated to dopamine in the brain by dopa decarboxylase, and it has beneficial effects produced through the actions of dopamine on D2 receptors (see Fig. 5.3). Dopamine itself is not used, owing to its inability to cross the blood–brain barrier.
Indications
L-dopa is used in the treatment of parkinsonism (excluding drug-induced extrapyramidal symptoms).
Adverse effects
Psychiatric side-effects are common limiting factors in l-dopa treatment; these include vivid dreams, confusion and psychotic symptoms more commonly seen in schizophrenia. These effects are probably a result of increased dopaminergic activity in the mesolimbic area of the brain, possibly similar to that found pathologically in schizophrenia (dopaminergic overactivity is implicated in schizophrenia, p. 82).
Dopamine agonists
Mechanism of action
Bromocriptine, ropinirole, cabergoline (longer acting), pergolide, pramipexole, lisuride and apomorphine are dopamine agonists selective for the D2 receptor (see Fig. 5.3). Apomorphine also has agonist action at D1 receptors. Pramipexole has a high affinity for D3 receptors.
Drugs stimulating release of dopamine
Amantadine is an example of a drug that stimulates the release of dopamine (see Fig. 5.3).
MAOB inhibitors
Selegiline is an example of a MAOB inhibitor.
Mechanism of action
Selegiline selectively inhibits the MAOB enzyme in the brain that is normally responsible for the degradation of dopamine (see Fig. 5.3). By reducing the catabolism of dopamine, the actions of l-dopa are potentiated, thus allowing the dose to be reduced by up to a third. There is evidence to suggest that selegiline may slow the progression of the underlying neuronal degeneration in Parkinson’s disease.
Drugs that inhibit striatal cholinergic activity
Anticholinergic agents
Benzatropine, procyclidine and orphenadrine are examples of anticholinergic (antimuscarinic) agents.
Mechanism of action
Benzatropine, procyclidine and orphenadrine are antagonists at the muscarinic receptors that mediate striatal cholinergic excitation (see Fig. 5.3). Their major action in the treatment of Parkinson’s disease is to reduce the excessive striatal cholinergic activity that characterizes the disease.
Sleep disorders and hypnotics
γ-Aminobutyric acid receptor
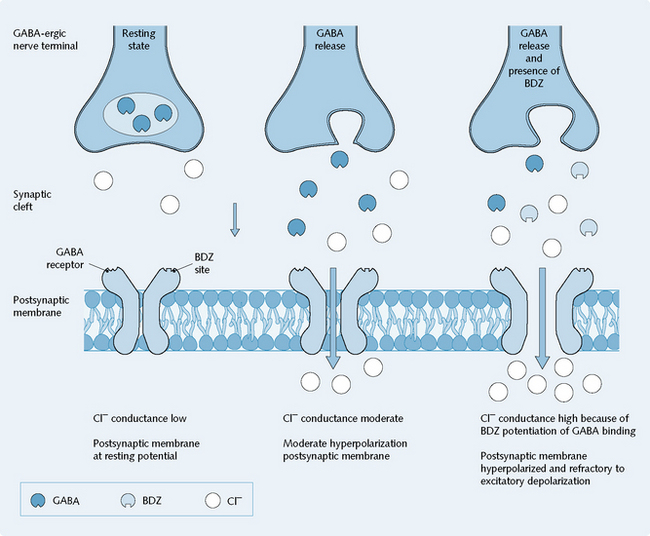
GABA released from nerve terminals binds to postsynaptic GABAA receptors, the activation of which increases the Cl– conductance of the neuron. Occupation of the benzodiazepine sites by benzodiazepine receptor agonists enhances the actions of GABA on the Cl– conductance of the neuronal membrane. The barbiturates similarly enhance the action of GABA, but by occupying a distinct modulatory site (Fig. 5.4).
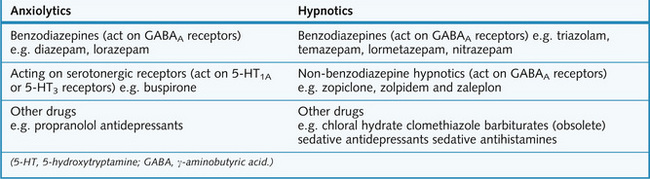
Anxiolytic and hypnotic drugs
The pharmacotherapy of anxiety and sleep disorders involves several different classes of drug, as shown in Figure 5.5, and non-pharmacological management relying on cognitive and behaviour psychotherapy.
Benzodiazepines
Mechanism of action
Benzodiazepines potentiate the action of GABA, the primary inhibitory neurotransmitter in the CNS. They do this by binding to a site on GABAA receptors, increasing their affinity for GABA. This results in an increased opening frequency of these ligand-gated Cl– channels, thus potentiating the effect of GABA release in terms of inhibitory effects on the postsynaptic cell (Fig. 5.4).
Route of administration
Oral is the usual route. Intravenous, intramuscular and rectal preparations are available.
Adverse effects
Benzodiazepines have several adverse effects:
Therapeutic notes
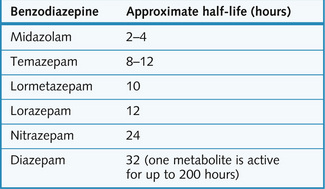
Benzodiazepines are active orally, and they differ mainly in respect of their duration of action (Fig. 5.6). Short-acting agents (e.g. lorazepam and temazepam) are metabolized to inactive compounds, and these are used mainly as sleeping pills because of the relative lack of ‘hangover’ effects in the morning. Some long-acting agents (e.g. diazepam) are converted to long-lasting active metabolites with a half-life longer than the administered parent drug. With others (e.g. nitrazepam) it is the parent drug itself that is metabolized slowly. Such drugs are more suitable for an anxiolytic effect maintained all day long, or when early morning waking is the problem.
Anxiolytic drugs acting at serotonergic receptors
Barbiturates
Barbiturates, however, still have a place in anaesthesia (p. 94) and to a lesser extent in the treatment of epilepsy (p. 94).
Miscellaneous agents
Antidepressants
If the underlying cause of insomnia is associated with depression, or particularly in depressed patients exhibiting anxiety and agitation, then tricyclic antidepressants (TCAs) with sedative actions (p. 79), e.g. amitriptyline, may be useful, as they act as hypnotics when given at bedtime. Alternatively, selective serotonin reuptake inhibitors (SSRIs, p. 80) may correct the mood disorder and lessen the symptoms of anxiety or insomnia.
Affective disorders
Monoamine theory of depression
The monoamine theory explains why:
The monoamine theory cannot explain why:
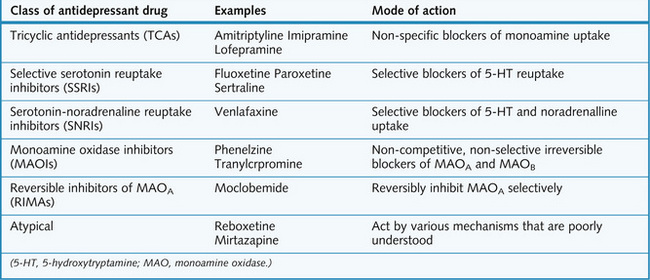
Treatment of unipolar depressive disorders
The major classes of drug that are used to treat depression, and their mechanisms of action, are summarized in Figure 5.7.
TCAs and related drugs
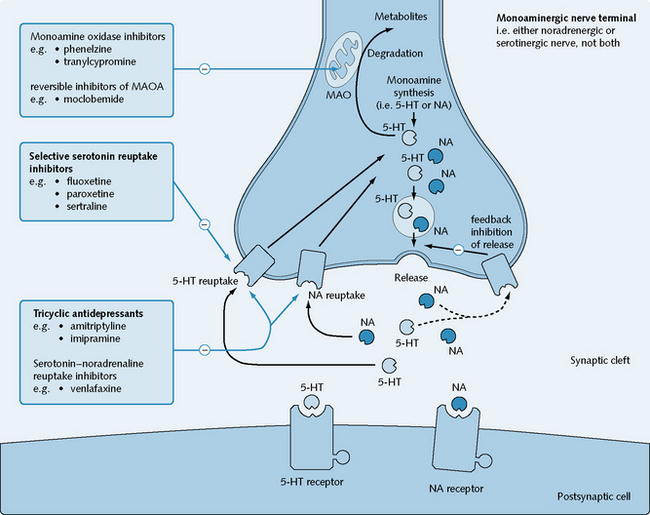
Mechanism of action
TCAs act by blocking 5-HT and noradrenaline uptake into the presynaptic terminal from the synaptic cleft (Fig. 5.8). They also have a certain affinity for H1 and muscarinic receptors, and for α1– and α2-receptors.
Contraindications
TCAs and related drugs should not be used in:
Selective serotonin reuptake inhibitors (SSRIs)
Mechanism of action
SSRIs act with a high specificity for potent inhibition of serotonin reuptake into nerve terminals from the synaptic cleft, while having only minimal effects on noradrenaline uptake (see Fig. 5.8). They block serotonin transporters, which belong to a class of Na+/Cl–-coupled transporters.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree