Some of the larger sulci are used to divide the surface of the hemisphere into lobes which are named according to the cranial bones that lie adjacent when the brain is in situ: frontal, temporal, parietal and occipital lobes. The frontal lobe lies in front of the central sulcus and above the lateral sulcus; the parietal lobe is behind the central sulcus and above the lateral sulcus; the temporal lobe is below the lateral sulcus; and the occipital lobe lies below and behind the parieto-occipital sulcus.
Superolateral surface
A deep fissure that separates the frontal and temporal lobes on the undersurface of the brain is continued to the superolateral surface and passes backwards, above the temporal lobe. This is the lateral sulcus (fissure of Sylvius) (Fig. 7.1), although strictly speaking the part on the lateral surface is the posterior ramus of the lateral sulcus, for, at the front end of this part, there are short anterior and ascending rami that branch off from it to penetrate the inferior frontal gyrus. The areas of cortex bounding the short sulci are the orbital, triangular and opercular parts of the inferior frontal gyrus. The parts of the frontal, parietal and temporal lobes that bound the lateral sulcus form the opercula, which overlie a buried part of the cortex, the insula (Fig. 7.2), composed of various long and short gyri almost completely surrounded by the circular sulcus.
 |
| Figure 7.2 Left cerebral hemisphere with the insula exposed by removal of the opercula. The diagram is based on a different brain to that depicted in Figure 7.1 and corresponding gyri and sulci are not identical. |
From just behind the midpoint of the superior border of the hemisphere, an oblique sulcus runs downwards and forwards to end a little above the lateral sulcus; it is the only long sulcus to pass over on to the medial surface of the hemisphere. This is the central sulcus (fissure of Rolando) and it separates frontal and parietal lobes (Fig. 7.1). The precentral and postcentral gyri lie in front of and behind it; they contain the primary motor and sensory cortical areas.
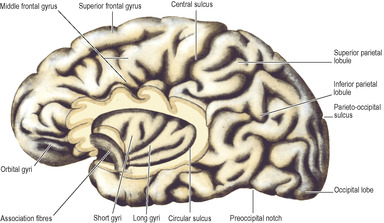
In front of the precentral gyrus the frontal lobe is divided by two horizontal sulci into three gyri, the superior, middle and inferior frontal gyri. A similar arrangement divides the temporal lobe below the lateral sulcus into superior, middle and inferior temporal gyri. The parietal lobe is divided by a transverse sulcus into superior and inferior parietal lobules. Into the latter project the lateral sulcus and the superior temporal sulcus; the posterior ends of these sulci are closed by the curved supramarginal and angular gyri respectively.
An imaginary line divides the occipital lobe from the parietal and temporal lobes. It extends from the small part of the parieto-occipital sulcus visible on this lateral surface, downwards in a 45° slope to the inferior border where there is often a slight preoccipital notch (Fig. 7.2) indented in the border by a fold of dura mater over the transverse sinus. A further arbitrary line, carried backwards from the main direction of the lateral sulcus until it meets the occipital demarcation line, indicates where the parietal and temporal lobes join.
Medial surface
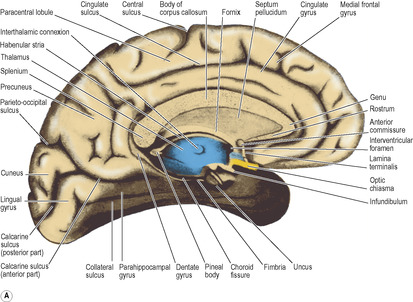
The two medial surfaces are flat and lie close together; they can be inspected only when their midline connections are divided by sagittal section (Fig. 7.3). Such a section severs the corpus callosum and the roof and floor of the third ventricle, as well as the brainstem and cerebellum. The medial surface of the hemisphere above the corpus callosum forms the cingulate gyrus, above which is the cingulate sulcus. The medial frontal gyrus lies above the anterior part of the sulcus and extends to the superior border of the hemisphere where it is continuous with the superior frontal gyrus. Just behind the midpoint of the superior border the central sulcus turns on to the medial surface; it is enclosed in the paracentral lobule.
At the posterior part of the hemisphere, the oblique parieto-occipital sulcus separates the parietal from the occipital lobe and extends over the superior border. The medial surface of the occipital lobe is wedge-shaped and is named the cuneus. Between the parieto-occipital sulcus and the paracentral lobule is the precuneus.
The cuneus is limited inferiorly by the calcarine sulcus which runs forward from the occipital pole to the medial surface of the temporal lobe. The visual area of the cortex occupies the lips of the sulcus (see p. 464). The parieto-occipital sulcus runs into it and the parieto-occipital and calcarine sulci form a pattern like the letter Y on its side; the common stem of the Y is the anterior part of the calcarine sulcus, and the two limbs are the parieto-occipital sulcus and the posterior part of the calcarine sulcus. The lingual gyrus lies below the posterior part of the calcarine sulcus and is limited at the border between the medial and inferior surfaces of the occipital lobe by the collateral sulcus.
Inferior surface
This shows the orbital surfaces of the frontal lobes and the sloping inferior surface of the temporo-occipital part of the brain (Fig. 7.4).
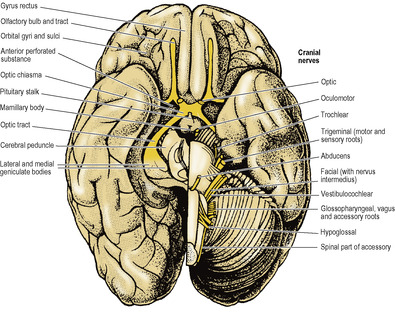
The orbital surface of the frontal lobe has the straight gyrus rectus along its medial margin. The olfactory bulb and the olfactory tract lie on the olfactory sulcus alongside the gyrus rectus. Lateral to the olfactory bulb and tract this surface is gently concave and is divided into a series of orbital gyri and sulci which leave prominent impressions on the orbital part of the frontal bone.
The temporal pole is boldly convex; the temporal lobe merges posteriorly with the occipital lobe and the continuous surface so formed is oblique in conformity with the slope of the tentorium cerebelli, against which it lies. Hence much of the medial surface of the temporal lobe can be seen from the inferior view. It is characterized by two long parallel sulci, the occipitotemporal sulcus laterally and the collateral sulcus medially (Fig. 7.3A). They run anteroposteriorly between the temporal and occipital poles. Medial to the collateral sulcus is the parahippocampal gyrus, confined to the temporal lobe and recurved at its anterior end to form the uncus (Fig. 7.3A); at the back it may appear to become continuous with the front of the lingual gyrus.
Internal structure
The interior of the forebrain is characterized by the presence within the white matter of large masses of grey matter and also by cavities which contain the CSF. The largest such mass of cells is the thalamus; it belongs to the diencephalon and is described on page 470.
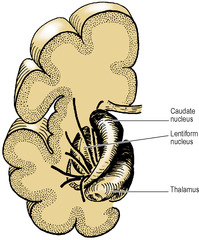
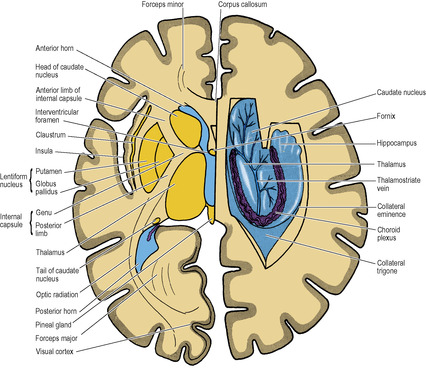
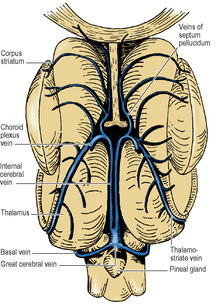
Other cell groups lie lateral to the thalamus within the cerebral hemisphere and some of them constitute the basal nuclei (also called basal ganglia). They are usually classified anatomically as consisting of the caudate nucleus, lentiform nucleus (which has an outer part, the putamen, and an inner part, the globus pallidus), amygdaloid body and claustrum. The caudate nucleus and the lentiform nucleus are separated by the internal capsule (see below). The caudate nucleus and the putamen part of the lentiform nucleus are joined by many interconnecting fibres, which pass through the anterior part of the internal capsule, giving the area a striated appearance. The caudate nucleus and lentiform nucleus together with the intervening internal capsule are hence referred to as the corpus striatum.
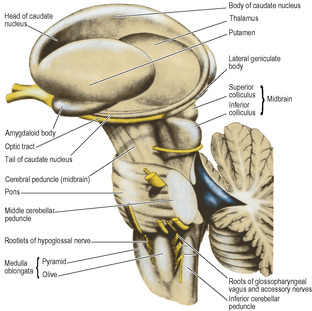
The caudate nucleus has the shape of a highly curved comma (Figs 7.5 and 7.6) with a head, body and tail. The bulbous head tapers back to the body which, curving back round the lateral part of the thalamus, bends sharply forwards into the long thin tail that joins the amygdaloid body. The caudate nucleus is curled snugly round the internal capsule like a hand holding a bunch of flowers. The whole length of its convexity projects into the lateral ventricle.
The lentiform nucleus is the shape of a biconvex lens, completely buried in the hemisphere (Figs 7.6 and 7.7). It is oval in outline and has two parts: the large lateral putamen and the small medial globus pallidus (or pallidum).
The amygdaloid body (or amygdala) consists of several groups of neurons and is connected with the tip of the tail of the caudate nucleus in the roof of the inferior horn of the lateral ventricle. The amygdala is functionally part of the limbic system (see p. 466). It has connections with the frontal and temporal lobes, including the olfactory cortex. An efferent bundle of axons, the stria terminalis, runs posteriorly following the curvature of the tail of the caudate nucleus, in the roof of the inferior horn and the floor of the body of the lateral ventricle, to the septal area and anterior hypothalamus.
The claustrum is a thin lamina, circular in outline and curved into a saucer-shape. It lies lateral to the putamen, and although easy to identify in horizontal or coronal sections (Fig. 7.7) its significance is unknown.
Functionally the basal nuclei exert a supraspinal control over skeletal muscle movements by influencing their rate, range and coordination. The corpus striatum receives fibres mainly from the cerebral cortex, thalamus and substantia nigra. The globus pallidus is the main efferent pathway from the corpus striatum and sends fibres to the thalamus, subthalamic nucleus, substantia nigra and the reticular formation. Different pathways involve different transmitters which include acetylcholine, dopamine, glutamate, serotonin and γ-aminobutyric acid (GABA). The most common disease of the basal nuclei is parkinsonism, characterized by tremor, rigidity and abnormal slowness of movements (bradykinesia); there is a decrease of dopamine in the nigrostriatal pathway.
The white matter of the cerebral hemisphere is made up of fibres belonging to three main groups.
Commissural fibres join the cortices of the two hemispheres. Most of them are gathered together in the corpus callosum; a few lie in the anterior and posterior commissures. They radiate widely and symmetrically through the white matter of the hemispheres.
Association (arcuate) fibres are confined to their own hemisphere, in which they connect different parts of the cortex.
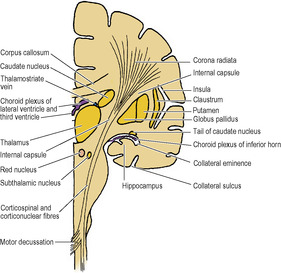
Projection fibres are those which join the grey matter of the hemisphere with subcortical nuclei in the hemispheres and with nuclei in the brainstem and spinal cord. In the base of the hemisphere a major collection of projection fibres lies lateral to the thalamus and the head of the caudate nucleus, forming the internal capsule. The lentiform nucleus lies lateral to the internal capsule. The tail of the caudate nucleus curls around the capsule and runs down to lie lateral to it (Fig. 7.5). From the internal capsule the fibres radiate upwards and outwards in the shape of a curved fan to reach the cortex and similarly pass from the cortex down to the capsule; this fan-shaped arrangement is the corona radiata. Fibres of the corpus callosum intersect it.
Internal capsule
The internal capsule consists of afferent fibres passing up to the cortex from cell bodies in the thalamus, and of efferent fibres passing down from cell bodies in the cortex to the cerebral peduncle of the midbrain. It lies within the concavity of the C-shaped caudate nucleus, which separates it from the C-shaped concavity of the lateral ventricle (Figs 7.5 and 7.7).
The internal capsule is seen in a typical horizontal section through the hemisphere (e.g. at a level through the interventricular foramen and the pineal gland) as a band of white matter that is not a straight line but bent into a lateral concavity by the convex medial border of the lentiform nucleus (i.e. by the globus pallidus). The internal capsule is thus described as having an anterior limb, genu and posterior limb, and there are also two other portions posteriorly: the sublentiform and retrolentiform parts.
The anterior limb lies between the head of the caudate nucleus medially and the lentiform nucleus laterally. It contains frontopontine fibres from cell bodies in the frontal cortex. They pass down below the thalamus into the cerebral peduncle, where they occupy the medial part of the base of the peduncle. They arborize round the pontine nuclei. Some thalamocortical fibres run through the anterior limb to the frontal cortex.
The genu is the region of the bend in the capsule (as seen in horizontal section), at the apex of the globus pallidus. Its principal constituents are the corticonuclear fibres which pass from the cerebral cortex to the motor nuclei of cranial nerves in the brainstem (see p. 481).
The posterior limb lies between the thalamus medially and the lentiform nucleus laterally. Occupying the anterior two-thirds of the posterior limb are the corticospinal fibres. From cell bodies in the cortex the fibres pass down through this part of the capsule, then through the brainstem to the lower medulla (Fig. 7.8) where most of them decussate to form the lateral corticospinal tract and eventually arborize with the anterior horn cells that innervate skeletal muscle. Thus passing through small parts of the internal capsules—genu and anterior two-thirds of the posterior limbs—are the motor fibres that control all the skeletal muscle in the body. The head (corticonuclear) fibres lie most anteriorly and immediately behind them are corticospinal fibres for the arm, hand, trunk, leg and perineum in that order from front to back. (In the cerebral peduncle of the midbrain the head fibres lie medially and the fibres for the perineum laterally, in the same order.) It is in this part of the internal capsule that haemorrhage or thrombosis of a striate artery commonly occurs. The muscles of the opposite side of the body are thus paralysed; they become spastic with increased stretch reflexes, the signs of an upper motor neuron lesion (see p. 491). Fibres from the speech (Broca’s) area are interrupted in lesions of the left internal capsule; thus loss of speech accompanies hemiplegia of the right side of the body.
Beside and behind the corticospinal fibres in the poste-rior limb of the capsule there are thalamocortical fibres passing from cell bodies in the thalamus to the cerebral cortex. These include sensory fibres mediating impulses derived from the opposite side of the body which run upwards through the corona radiata to the sensory cortex. There are also some frontopontine fibres.
In the retrolentiform part of the capsule, at the posterior end of the lentiform nucleus, are parieto- and occipitopontine (corticopontine) fibres which will occupy the lateral part of the base of the cerebral peduncle. This part of the capsule also contains visual fibres passing from cell bodies in the lateral geniculate body to the visual area of the cortex as the optic radiation (Fig. 7.7). A further group of fibres runs from the medial geniculate body below the posterior end of the lentiform nucleus, so forming the sublentiform part of the capsule. These are the fibres of the auditory radiation which reach the auditory area of the cortex in the anterior transverse temporal gyrus (see p. 463). Temporopontine fibres also pass through the sublentiform part.
Corpus callosum
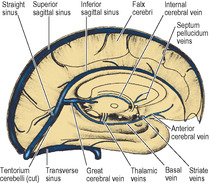
The corpus callosum (Fig. 7.3) consists of a mass of 100 million commissural fibres, each of which extends from cortex to cortex between symmetrical parts of the two hemispheres. It commences at the anterior commissure, at the upper end of the lamina terminalis of the diencephalon and, traced from here to its termination, it becomes increasingly thicker. It is described as having four parts: the rostrum, genu, body and splenium. From the anterior commissure the mass passes upwards and forwards as the rostrum. It now takes a sharp bend back-wards as the genu. From here it is gently convex upwards (the body) and it ends posteriorly as a thick rounded free border, the splenium. The corpus callosum can be seen by separating the two hemispheres, and its cut surface is exposed in a midline sagittal section through the brain (Fig. 7.12).
The fibres of the corpus callosum extend to all parts of the cerebral cortex. In a horizontal section the fibres of the genu are seen arching forwards on each side to the frontal cortex; this appearance gives them the name forceps minor. Similarly, the fibres of the massive splenium curve backwards symmetrically to the occipital cortex, forming the forceps major (Fig. 7.7).
Between forceps minor and forceps major the fibres of the corpus callosum spread out to the cortex on the lateral surface of the hemisphere. They pass over the anterior horn and body of the lateral ventricle, for each of which they form the roof. As they turn down into the temporal lobe they form the lateral wall of the inferior and posterior horns of the lateral ventricle, where they are known as the tapetum.
Cortical areas
Certain areas of the cerebral cortex have long been identified with specific functions. Although these areas are still clinically relevant, modern investigations are modifying traditional concepts as far as the separation of motor and sensory functions is concerned. Many motor fibres, for example, have their origin outside the traditional motor cortex and some arise from what were previously regarded as purely sensory areas. A new terminology has emerged, and it is now customary to refer to a combined ‘sensorimotor cortex’, subdividing it into four areas designated by the letters Ms and Sm (the capital M or S indicating whether the association is predominantly with motor or sensory functions). Thus the area MsI (first or primary motosensory area) includes the old ‘motor and premotor’ regions of the precentral and other gyri of the frontal lobe (corresponding to areas 4 and 6 as described by Brodmann in his classical study of cortical histology). The area MsII (the supplementary motor area) is on part of the medial surface of the frontal lobe (part of areas 6 and 8). Similarly SmI (first sensorimotor area) includes most of the postcentral gyrus (areas 3, 1 and 2) and its extension on to the medial surface of the parietal lobe, and SmII is the lowest part of the postcentral gyrus (areas 40 and 43). These four main motor and sensory areas have many interconnections, both within their own and with the opposite hemisphere.
The area MsI is where movements of the various parts of the body are initiated, and it receives its main inputs from the cerebellum and thalamus. Some of the cortical cells send their axons down as the corticonuclear and corticospinal (pyramidal) tracts (see p. 481). MsII receives many fibres from thalamic nuclei which in turn have received fibres from the basal nuclei; its role in the control of movements is mainly with the mental processes that precede the effecting of a movement. In the precentral gyrus of MsI the body is represented upside down along this cortex, although the face itself is represented the right way up. The face lies lowest, then the hand (a very large area), then arm, trunk and leg. The leg overlaps the superior border and the foot and perineum extend down on the medial surface of the hemisphere into the paracentral lobule. In MsII the body is represented with the face anterior, the lower limbs posterior and the upper limbs in between.
The motor (anterior) speech area (of Broca, areas 44 and 45) is usually situated in the inferior frontal gyrus on the left side (in right-handed and in most left-handed people), below and in front of the face area and centred on the triangular part between the anterior and ascending rami of the lateral fissure. Damage to it produces motor aphasia: difficulty in finding the right words but not paralysis of laryngeal musculature.
The posterior speech area (of Wernicke) is in the posterior parts of the superior and middle temporal gyri and extends into the lower part of the parietal lobe. Its integrity is necessary for the understanding of speech.
The frontal eye field, involved in voluntary eye movements is in the centre of the middle frontal gyrus (parts of areas 6, 8 and 9).
The areas SmI and SmII receive a large thalamic input. SmI is for the appreciation of touch, kinaesthetic and vibration sense, and the parts of the body are represented in roughly the same way as in MsI and MsII. SmII appears to be associated with pain and temperature sensations. Although the conscious appreciation of pain may occur at the thalamic level, the cortex is necessary for its localization.
The gustatory area for the conscious appreciation of taste lies in the inferior part of the postcentral gyrus (frontoparietal operculum), near the tongue area of SmI.
The auditory area (areas 41 and 42) is mostly in the floor of the lateral sulcus, in the anterior transverse temporal gyrus. It extends into the superior temporal gyrus below the sulcus, and is here surrounded by the auditory association area (area 22). These regions receive fibres from the medial geniculate body via the auditory radiation. The cochleae are bilaterally represented, so a lesion of one cortex does not cause complete unilateral deafness.
The olfactory area is in the uncus at the front of the parahippocampal gyrus (Fig. 7.3A) and in adjacent parts of the cortex.
The visual area (area 17) is mainly on the medial surface of the occipital lobe on the lips of the posterior part of the calcarine sulcus, and extends for a short distance on to the lateral surface of the occipital lobe as far as the lunate sulcus, a small curved sulcus in front of the occipital pole (Fig. 7.1). The true visual area is characterized by a white line (stria of Gennari) which bisects the grey matter of the cortex; in cortical sections it is easily seen with the naked eye, hence the name ‘striate cortex’ often given to this area. The cortex adjacent to the striate part on the medial and lateral surfaces of the hemisphere forms the visual association area (areas 18 and 19).
Each visual area receives from its own half of each retina, i.e. it registers the opposite visual field, on account of the curved configuration of the retina and the crossing of the medial fibres of the optic nerve at the optic chiasma. The temporal (lateral) half of the visual field of one eye conveys its impressions to the nasal (medial) half of the retina of that eye; similarly the temporal half of the retina receives its impressions from the nasal half of the visual field. In each cortex the upper half receives from the upper half of each half-retina, the lower half from the lower half of each half-retina; accordingly the representation of the upper and lower halves of the visual fields is reversed on the cortex. The macula registers at the posterior end of the visual area over a disproportionately large area, while more peripheral parts of the retina register progressively more anteriorly.
Cortical structure
The cerebral cortex is composed of layers of cells which vary in their characteristics in different regions. In most parts of the cortex six layers of nerve cells can be distinguished, and are conventionally numbered from the surface inwards by Roman numerals. Layer I has an abundance of fibres with relatively few cells, the plexiform layer. Then follow the external granular (II), external pyramidal (III), internal granular (IV), internal pyramidal (ganglionic) (V) and multiform (VI) layers, roughly named from the density and shapes of their cells. In layer IV there are often prominent strands of horizontal fibres, and in the visual cortex they form the stria of Gennari.
Changes in the relative distribution of these layers are most pronounced in the known sensory and motor areas. The postcentral gyrus (touch), the superior temporal gyrus (hearing), and the calcarine sulcus (sight) are covered by cortex in which granular cells predominate, while motor areas typically have larger numbers of pyramidal cells. Among the cells of layer V of the precentral gyrus are the giant pyramidal cells (of Betz), which resemble large anterior horn cells of the spinal cord; they give rise to no more than 2% of corticospinal fibres.
The white matter is composed of myelinated nerve fibres bound together by the fibres of the neuroglia. Myelin within the central nervous system is derived from the oligodendrocytes (in contrast to the peripheral nervous system where it comes from Schwann cells).
Visual pathways
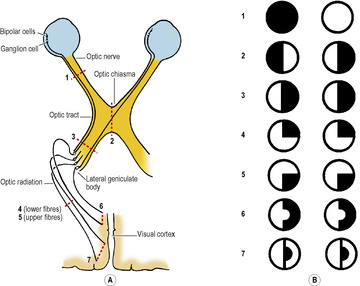
The peripheral nerves of ordinary sensation, with their cell bodies in posterior root ganglia, are represented in the visual pathway by the bipolar cells of the retina (Fig. 7.9A). These cells receive impulses from the retinal rods and cones. The bipolar cells synapse with ganglion cells in the inner part of the retina (next to the vitreous body, 1 cell for each cone, 1 cell for 80 rods). These are homologous with the second neuron cell bodies in the central nervous system in the other sensory pathways. Their axons run on the inner surface of the retina and enter the optic disc and so pass to the optic nerve.
The optic nerve is not a nerve in the sense of other cranial and spinal nerves; it is an elongated tract of white matter stretched out from the brain and enclosed in the meninges thereof as far forward as its attachment to the sclera. Histologically it is identical with white matter of the central nervous system, and there is no effective regeneration when divided. In the orbit it is surrounded by a tube of dura mater and arachnoid, with CSF in the subarachnoid space. At the optic foramen the dura and arachnoid leave it and the nerve, still sheathed in pia mater, passes up to meet its fellow at the optic chiasma, which is attached to the anterior part of the floor of the third ventricle.
In the chiasma the nasal fibres of each optic nerve decussate and pass into the optic tract of the opposite side; as they do so, some crossing fibres loop forwards slightly into the contralateral optic nerve before entering the optic tract, while some fibres loop backwards slightly into the ipsilateral optic tract before crossing the midline. The temporal fibres from each retina pass directly to the optic tract of their own side (Fig. 7.9A). Thus the right optic tract contains fibres from the right half of each retina, i.e. it carries impressions from the nasal field of the right eye and the temporal field of the left eye. Likewise, the left optic tract contains fibres which carry impressions from the right half of each visual field.
Cortical pathways for common sensation consist of three neurons. They reach the opposite hemisphere by a complete decussation of the second order neurons. The visual pathway by the half decussation of its second order neurons at the chiasma achieves the same object. One hemisphere registers common sensation from the opposite half of the body and also from the opposite half of the visible environment.
The optic tract passes from the chiasma around the cerebral peduncle, high up against the temporal lobe, and, reaching the side of the thalamus, divides into two branches. The larger of these enters the lateral geniculate body, in which the fibres synapse. These are visual fibres. The smaller branch (superior brachium) passes down medially, between the lateral and medial geniculate bodies, and synapses in the superior colliculus and the pretectal nuclei; these are fibres mediating light reflexes (see p. 407).
Blood supply. The optic tract is supplied chiefly by the anterior choroidal and posterior communicating arteries, the chiasma and intracranial part of the optic nerve by the anterior cerebral. In the orbit the nerve is supplied by the ophthalmic artery and, distally, by the central artery on its way to the retina.
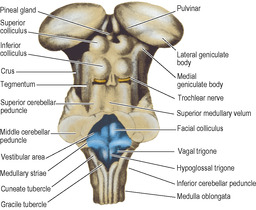
The lateral geniculate body, which is a part of the thalamus, is a small rounded elevation on the posterior surface of the thalamus (Figs 7.4 and 7.20). It has six layers of neurons numbered 1–6 from the ventral to the dorsal surface. Crossed optic tract fibres end in layers 1, 4 and 6; uncrossed fibres end in layers 2, 3 and 5. The neurons of the geniculate body send their axons through the optic radiation to the occipital cortex (Fig. 7.9). The axons from the lateral part of the geniculate body, carrying impressions from the upper part of the opposite side of the visual fields, fan out laterally and inferiorly around the anterior tip of the inferior horn of the lateral ventricle (Meyer’s loop) before swinging posteriorly to reach the inferior lip of the calcarine sulcus; axons from the medial part, carrying impressions from the corresponding inferior part of the visual fields, pass directly backwards to the superior lip of the calcarine sulcus.
 |
| Figure 7.20 |
The superior brachium is the small (medial) branch of the optic tract. It passes down to the tectum of the midbrain, (see p. 476), where its fibres synapse with cells in the superior colliculus. Tectobulbar and tectospinal tracts from the superior colliculus pass to motor nuclei in the brainstem and spinal cord for the mediation of general light reflexes (e.g. reflex blinking and jumping or turning away from a flash of bright light). The superior colliculi are united by the posterior commissure and thus general body reflexes to light are usually bilateral. The fibres concerned in the pupillary light reflex (see p. 407) do not synapse in the colliculus, but pass bilaterally to each pretectal nucleus. The pretectal nucleus is a small group of cells in the tegmentum of the midbrain, just cranial to the superior colliculus. It passes light impulses to each Edinger–Westphal nucleus and so to the sphincter pupillae. A lesion here produces the Argyll Robertson pupil; contraction to light is lost, but the pupil still contracts to accommodation and convergence.
Limbic system and olfactory pathways
In the vicinity of the corpus callosum and diencephalon are a number of features that have come to be known collectively as the limbic system. Because the olfactory tracts and its associated structures were originally included in this descriptive concept, much of its function was thought to be concerned with olfaction. This view is no longer tenable and it is now known to play a role in such abstract functions as emotion, behaviour, mood and memory; thus lesions of one of its major constituents, the hippocampus, result in loss of memory for recent events, although the memory of distant events is retained. However, much remains to be discovered about the form and function of the limbic system.
Limbic system
Apart from the olfactory nerves, bulb and tract, the following are among the major components of the limbic system:
Olfactory pathways
The olfactory tract, which is an elongated extension of the white matter of the brain (like the optic nerve), lies in the olfactory sulcus beside the gyrus rectus on the inferior surface of the frontal lobe (Fig. 7.4). Its anterior end is expanded as the olfactory bulb. After passing through the cribriform plate of the ethmoid, the olfactory nerve filaments synapse with the cells of the second order neuron in the bulb. The axons of these cells run back in the tract to the anterior perforated substance, which lies on the inferior surface of the frontal lobe immediately lateral to the optic chiasma. Here the tract divides into lateral and medial striae. The lateral stria runs along the anterolateral margin of the anterior perforated substance to the region of the uncus, at the front of the parahippocampal gyrus (Fig. 7.3). The medial stria passes in front of the lamina terminalis and makes connections with other parts of the limbic system.
Thus the olfactory pathway reaches the olfactory cortex without relay in the thalamus, unlike other sensory pathways (light, sound, taste, touch). However, the olfactory cortex projects directly and via the thalamus to areas of the orbitofrontal cortex that are involved in olfactory information processing. Furthermore, connections with the hypothalamus and brainstem mediate visceral and somatic effects distinct from conscious perception.
Hippocampus
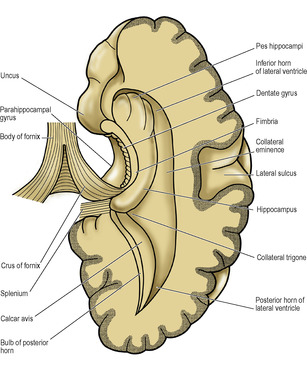
Just above the anterior part of the parahippocampal gyrus of the temporal lobe lies the hippocampal sulcus, which is projected into the floor of the inferior horn of the lateral ventricle as the hippocampus. The transitional zone between the cortex of the parahippocampal gyrus and that of the hippocampus is known as the subiculum. Viewed from above the anterior part of the hippocampus (the pes hippocampi) has the appearance of the knuckles of a clenched fist (Figs 7.7 and 7.10). On its ventricular surface is a thin film of white matter, the alveus; its cell bodies are in the hippocampus and subiculum. The fibres of the alveus thicken medially to form the fimbria. This breaks free from the hippocampus as the crus (posterior pillar) of the fornix (Fig. 7.10). The dentate gyrus is a small part of the hippocampus which, as seen from the medial side, lies between the fimbria and the parahippocampal gyrus.
Fornix
The fornix is the great efferent pathway from the hippocampus. As a flat band continuous with the fimbria, it curves up behind the thalamus to join its fellow in a partial decussation across the midline, the commissure of the fornix. It is really a chiasma, and is an association tract as well as a commissure. The conjoined mass of white matter, lying beneath the corpus callosum, is the body of the fornix. From it the conjoined anterior columns arch down in front of the anterior poles of the thalami and diverge, forming the anterior margins of the interventricular foramina (Fig. 7.12).
Fibres from the anterior column of the fornix pass both anterior and posterior to the anterior commissure. The anterior fibres pass mainly to the septal nuclei near the lamina terminalis. The posterior fibres pass directly to the thalamus or into the mamillary body. From the mamillary body fibres pass in the lateral wall of the third ventricle as the mamillo-thalamic tract to the anterior nucleus of the thalamus. Here they relay and the thalamic neurons send their fibres through the internal capsule to the cingulate gyrus, which has connections with the parahippocampal gyrus and thus to the hippocampus.
Lateral ventricles
Each lateral ventricle is a C-shaped cavity, lined with ependyma, lying within the cerebral hemisphere. It consists of the body of the ventricle and anterior, posterior and inferior horns (Figs 7.7 and 7.11). On its medial side the pia mater and ependyma come into contact with each other. This line of contact is narrow, and curves around the top of the thalamus and the tail of the caudate nucleus, forming a C-shaped slit on the medial surface of the hemisphere, the choroid fissure (Fig. 7.3). The choroid fissure should be regarded as the medial wall of the body and inferior horn of the ventricle. A mass of blood capillaries enters through the choroid fissure, invaginating the pia mater and ependyma before it. This combination of capillaries, pia and ependyma constitutes the choroid plexus, which secretes CSF. The lips of the choroid fissure meet around the invaginated plexus, which thus lies hidden within the body and inferior horn of the ventricle. The choroid plexuses of the lateral ventricles are large and highly vascular; this pair secretes the bulk of the CSF. Each lateral ventricle opens into the third ventricle by the interventricular foramen, and the choroid plexus of the lateral ventricle is continuous through this foramen with the very small amount of plexus in the third ventricle.
In other places the grey matter at the bottom of a sulcus indents the cavity of the lateral ventricle. Such sulci are the parahippocampal, calcarine and collateral, which show as convexities within the cavity of the ventricle. The caudate nucleus and thalamus also project into the cavity. Elsewhere the walls of the cavity are formed by white matter of the cerebral hemisphere.
The anterior horn is bounded by the fibres of the corpus callosum that run laterally from the genu (forceps minor). The bulbous head of the caudate nucleus lies in the floor, meeting the roof at an angle on the lateral side (Fig. 7.7), but separated from the roof medially by a thin partition between the fornix and corpus callosum, the septum pellucidum (Fig. 7.12). Behind the anterior column of the fornix, between it and the anterior pole of the thalamus, is a small aperture, the interventricular foramen (of Monro), which leads from the lateral into the third ventricle. The choroid plexus in the body of the ventricle does not extend into the anterior horn but passes through the interventricular foramen into the third ventricle.
The body of the lateral ventricle lies behind the level of the interventricular foramen. Its floor is the thalamus and body of the caudate nucleus, with the thalamostriate groove between them. The stria terminalis lies in the groove (see p. 459). Also in the groove is the thalamostriate vein (Figs 7.7 and 7.17). The roof of the body is the corpus callosum with, on the medial side, the body and crus of the fornix (Fig. 7.12). Through the medial wall of the body of the ventricle, between roof (fornix) and floor (thalamus) the choroid plexus is invaginated, thrusting pia mater and ependyma before it. This is the upper part of the choroid fissure which is limited anteriorly by the interventricular foramen.
From the body the cavity of the lateral ventricle arches downwards and then forwards into the temporal lobe as the inferior horn, and backwards into the occipital lobe as the posterior horn.
The posterior horn is the most variably developed and may even be absent. The floor is a convexity, the collateral eminence, produced by the collateral sulcus. The medial wall consists of two slight convexities: the upper is the bulb of the posterior horn, formed by fibres of the forceps major (from the splenium of the corpus callosum), and the lower is the calcar avis, formed by the calcarine sulcus (Fig. 7.10). It is the calcar which, if well developed, obliterates the posterior horn. The roof and lateral wall is formed by the tapetum of the corpus callosum, with the optic radiation lying against the tapetum in the lateral wall (Fig. 7.7).
The inferior horn is the largest. Its floor consists medially of the hippocampus with, laterally, the collateral eminence, which expands posteriorly into the collateral trigone where the posterior and inferior horns diverge (Figs 7.7 and 7.10). In the roof is the tail of the caudate nucleus. The fimbria forms the lower lip of the choroid fissure in the inferior horn just as its continuation the fornix forms the upper lip of the fissure in the body of the lateral ventricle; they lie in continuity around the convexity of the C. Similarly, within the concavity of the C the caudate nucleus lies in continuity. Its bulbous head in the anterior horn and its thinner body in the floor of the body of the ventricle are continued into the roof of the inferior horn as an ever-diminishing tail of the caudate nucleus. At the extremity of the tail of the caudate nucleus is an expansion of grey matter, the amygdaloid body, which lies to the lateral side of the anterior perforated substance. It produces a shallow convexity on the roof at the tip of the inferior horn, just above the pes hippocampi. These two projections often lie together, separated only by the choroid plexus. The inferior horn is closed laterally by the white matter of the tapetum.
Diencephalon and third ventricle
That part of the brain cranial to the midbrain is the forebrain. Developed as a single tube (the fore-end of the neural tube) its cranial end is formed by a thin plate of grey matter, the lamina terminalis. Just to the caudal side of this lamina the side walls of the forebrain blow out into two enormous balloons, or vesicles, which become the cerebral hemispheres, already described. The remainder of the forebrain, relatively unexpanded, becomes the diencephalon, still closed anteriorly by the lamina terminalis. The cavity within its substance is the third ventricle, into which the lateral ventricles of the cerebral hemispheres open through the interventricular foramina. The diencephalon, enclosing this cavity, has two side walls, a floor and a roof. The floor and roof converge towards each other posteriorly, where they join the midbrain; and the cavity of the third ventricle is continued through the midbrain as the narrow aqueduct.
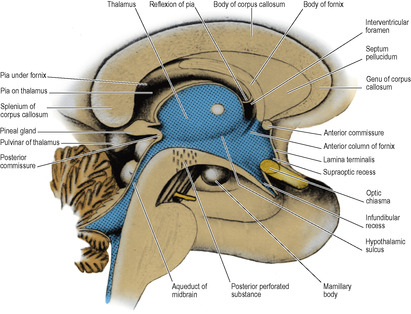
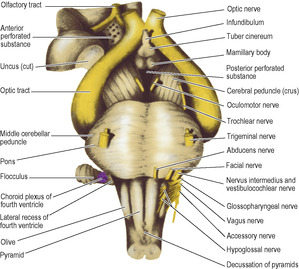
The anterior wall of the diencephalon is the lamina terminalis, a thin sheet which extends between the two hemispheres from the rostrum of the corpus callosum to the top of the optic chiasma (Fig. 7.12). The floor, seen from below as the floor of the third ventricle, is formed by the optic chiasma, tuber cinereum and infundibulum, mamillary bodies and the posterior perforated substance, where the floor joins the tegmentum of the cerebral peduncles (Fig. 7.18).
In a median sagittal section through the third ventricle a thin partition, the septum pellucidum, is seen connecting the rostrum, genu and front of the body of the corpus callosum to the anterior column of the fornix (Fig. 7.12). The septum consists of two layers that may be adherent; when they lie apart the closed space between them is the cavity of the septum pellucidum, which is lined with pia mater and has no connection with the ventricular system.
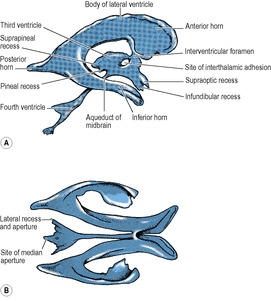
The third ventricle (Fig. 7.12) is a slit-like space, lying in the sagittal plane below the fornix and the corpus callosum. Much of the lateral wall is occupied by the thalamus, a rounded mass of grey matter that bulges convexly into the ventricle. The two thalami often (60% of brains) become gummed together at the interthalamic adhesion. It is not a commissure and there is no interchange of fibres between the two sides. The adhesion, when present, gives a fenestrated shadow in images of the third ventricle. A faint groove on the lateral wall, the hypothalamic sulcus, curves down from the interventricular foramen below the thalamus towards the aqueduct of the midbrain.
Below the hypothalamic groove the side wall slopes down to the floor. This region, including the floor, is the hypothalamus (Fig. 7.12). The caudal part of this area that merges with the midbrain is the subthalamus, one of whose principal features is the subthalamic nucleus which belongs functionally to the basal nuclei.
The hypothalamus contains various cell groups, in particular the supraoptic and paraventricular nuclei whose axons run in the pituitary stalk into the posterior lobe of the pituitary (see p. 448), and other cells whose processes enter the pituitary stalk to deliver their neurosecretory material to the hypothalamo–hypophyseal portal system of blood vessels for the control of the anterior lobe. Yet other cells have long axons that pass through the brainstem and spinal cord to preganglionic sympathetic cells in the lateral horn of the thoracic and upper lumbar parts, and to preganglionic parasympathetic cells in the lateral horn of the sacral segments, of the spinal cord (see p. 18).
Behind the optic chiasma the (hollow) infundibulum projects downwards to become the (solid) pituitary stalk. Behind the infundibulum the upper surface of the floor slopes smoothly upwards and backwards to the aqueduct, but the external surface is marked by the pair of mamillary bodies and behind them by the posterior perforated substance. The part of the floor between the optic chiasma and the mamillary bodies is the tuber cinereum. The part of the tuber cinereum at the base of the infundibulum is the median eminence which is the site of the neurosecretory cells that control the anterior pituitary, and one of the few regions with no blood–brain barrier (see p. 472).
The anterior wall of the third ventricle is the lamina terminalis which extends up from the optic chiasma to the rostrum of the corpus callosum. The tiny angle between the lamina terminalis and the chiasma is the supraoptic recess, and the hollow in the infundibulum is the infundibular recess (Fig. 7.12). Attached behind the upper end of the lamina terminalis is a rounded cord, the anterior commissure, which joins the inferior parts of the temporal lobes. Behind this the conjoined anterior columns of the fornix lie in contact before they diverge to sink down into the lateral wall of the ventricle. Behind each anterior column is an interventricular foramen, ependyma-lined and roofed in by bare pia mater sweeping from the under-surface of the body of the fornix to the upper surface of the anterior pole of the thalamus.
The roof of the third ventricle is slack towards its posterior end and bulges back as the suprapineal recess (Fig. 7.11). The whole length of the roof is invaginated by the pair of (small) choroid plexuses of the third ventricle, which hang down as slender fringes inside the cavity.
The pineal gland projects back from the posterior wall of the third ventricle, lying above the superior colliculi of the midbrain, between the posterior parts of the thalami, just below the splenium (Figs 7.12 and 7.20). It is a soft conical body, less than half a centimetre long, and is one of the few regions with no blood–brain barrier (see p. 472). It contains a number of corpora arenacea. These calcify, and to such an extent that after the age of 40 years they may throw a tiny shadow in radiographs of the skull. A displaced calcified pineal indicates a space-occupying lesion above the tentorium. Pineal secretions, including melatonin, have an inhibitory effect on other endocrine organs and gonads. The stalk of the pineal is attached also to the posterior commissure, which connects the two superior colliculi above the entrance to the aqueduct. The pineal stalk is hollowed out as the pineal recess (Fig. 7.11).
The thalamus is seen in horizontal and coronal sections buried by the cerebral hemisphere (Figs 7.7 and 7.8), and by its connections with the sensory parts of the internal capsule it appears to be part of the hemisphere. Nevertheless, it is part of the lateral wall of the diencephalon.
The mass of grey matter making up the thalamus is roughly wedge-shaped. The medial walls of the two thalami lie parallel, near each other across the third ventricle, where in two-thirds of cases they are joined by the interthalamic adhesion. Behind this the medial surface diverges from the midline and expands into a large posterior convexity, the pulvinar; the lateral geniculate body (see p. 465) bulges down from its lateral part (Figs 7.4 and 7.20). The medial geniculate body, a thalamic nucleus which relays auditory impulses, is separated from the main mass of the thalamus and lies on the midbrain. It receives fibres from the cochlear nerves by way of the nuclei of the nerves and the lateral lemniscus, and relays them through the sublentiform part of the internal capsule to the auditory cortex in the temporal lobe.
The superior surface of the thalamus is convex and triangular in outline, tapering forward from the large pulvinar to the small blunt anterior pole. The superior surface and the posterior surface (pulvinar) of the thalamus are on the external surface of the diencephalon itself. They are covered in pia mater. An oblique strip along the lateral margin of the superior surface lies in the lateral ventricle, covered with ependyma. The body and tail of the caudate nucleus are in contact here with the lateral margin of the thalamus (Fig. 7.6).
The lateral surface of the thalamus is bevelled by the internal capsule (Fig. 7.8), whose descending fibres lie in contact anteriorly. The ascending fibres of the internal capsule arise further back from numerous nuclei in this lateral part.
The inferior surface of the thalamus is narrower than the superior surface. Medially it joins the hypothalamus. Lateral to this, and posterior too, the lemnisci of the tegmentum (see p. 477) enter the thalamus and attach it to the top of the midbrain.
All four surfaces (medial, inferior, lateral and superior) of the thalamus converge to the small blunt anterior pole, which lies at the interventricular foramen, covered in ependyma.
Structurally the thalamus consists of a large number of cell groups, the thalamic nuclei, which receive inputs from the medial, spinal and trigeminal lemnisci and the reticular formation and project to sensory areas of the cerebral cortex. Other nuclei receive fibres from the dentate nucleus of the cerebellum and the globus pallidus and project to the motor areas of the cortex, so contributing to motor control. Some nuclei receive fibres from the hypothalamus and corpus striatum and have reciprocal connections with the frontal lobes; they appear to be concerned with emotional responses and memory. Via the mamillothalamic tract the hypothalamus also sends fibres to the anterior pole of the thalamus, which project to the cingulate gyrus and are part of the limbic system. The medial and lateral geniculate bodies are specialized parts of the thalamus concerned with hearing and vision respectively.
Blood supply of the forebrain
The cerebral hemispheres and the walls of the diencepha-lon are supplied from both the internal carotid and vertebral systems. The arteries are directed in essence to the grey matter, which needs more blood than the white matter. Superficial cortical arteries supply the grey matter on the surface, perforating arteries supply the subcortical nuclei. Both sets of arteries send branches to the adjacent white matter.
An artery that has entered the surface of the brain from either of these sets is always an end artery (i.e. it has no precapillary anastomosis with its fellows), and thus cerebral softening follows its obstruction. Entering arteries invaginate a tubular prolongation of pia mater around them, forming a perivascular space that extends to the fine branches of the vessel.
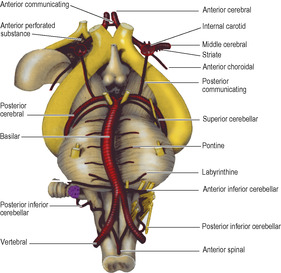
The internal carotid and vertebral systems anastomose with each other around the optic chiasma and infundibulum of the pituitary stalk, forming the arterial circle of Willis (the French call it, more accurately, the polygon of Willis). The communicating vessels allow equalization of blood flow between the two sides of the brain, and can allow anastomatic circulation if parts are occluded; however, this is not always effective due to the small size of some vessels. The circle is formed in the following way. The basilar artery from the vertebral system divides at the upper border of the pons into right and left posterior cerebral arteries. Each posterior cerebral receives a small posterior communicating artery that runs backwards through the interpeduncular cistern from the internal carotid artery at the anterior perforated substance on the same side. Each internal carotid artery gives off an anterior cerebral artery; the circle of Willis is completed by the anterior communicating artery, a small vessel that unites the anterior cerebrals in the chiasmatic cistern, below the rostrum of the corpus callosum. The optic chiasma and the pituitary stalk are encircled by the circle of Willis (Fig. 7.19). Rupture of an aneurysm of the arterial circle accounts for 90% of subarachnoid haemorrhages. Congenital aneurysms are more commonly found on the carotid part of the circle than the basilar part, and are most frequent at junctional sites where vessels branch (e.g. anterior cerebral with anterior communicating, internal carotid with posterior communicating or middle cerebral) because here the tunica media is weakest.
Blood supply of the cerebral hemispheres
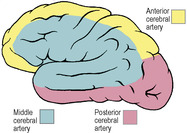
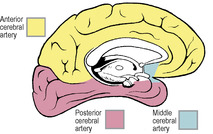
The arterial supply of the cerebrum is by the three cerebral arteries, anterior, middle and posterior and there is also a contribution from the anterior choroidal (although, unlike the other three, it does not supply the cerebral cortex). The first two and the last are branches of the internal carotid, the posterior cerebral is the terminal branch of the basilar. The branches of the three cerebral arteries anastomose across the frontiers of their respective territories (Figs 7.13 and 7.14), on the surface of the pia mater, but sparsely and only by arterioles. Their perforating branches are invariably end arteries. The larger surface vessels have a sympathetic innervation, but after becoming intracortical they are not innervated.
Capillaries in the brain (and spinal cord) are characterized by lack of fenestrations and by abundant tight junctions (zonulae occludentes) between endothelial cells. This is the principal structural reason for the blood–brain barrier which operates to protect the internal environ-ment of neural tissue by allowing only selected substances (amino acids, amines and sugars) to be transported across the endothelial cells. This protective mechanism is possibly assisted by a basal lamina that is thicker than usual and by the enveloping foot processes of astrocytes. Among the more important parts of the brain that have no blood–brain barrier are the posterior pituitary, median eminence of the hypothalamus and pineal gland.
The internal carotid artery emerges from the roof of the cavernous sinus, gives off the ophthalmic artery, then curls back to lie on the front half of the roof. It then turns vertically upwards to the anterior perforated substance where it divides into middle and anterior cerebral branches for the supply of the cortex (see Figs 6.106 and 6.107, p. 449). It here gives off also the striate arteries, the anterior choroidal artery, and the posterior communicating artery (Fig. 7.19).
The middle cerebral artery is the largest and most direct branch of the internal carotid (Fig. 7.19) and therefore most subject to embolism. It passes deep into the lateral sulcus to supply the cortex of the insula and overlying opercula. It reaches the lateral surface of the hemisphere by continuing in the lateral sulcus, from which its branches emerge and ramify over an area that falls short of the borders of the lateral surface by one gyrus or its equivalent breadth (Fig. 7.13). In its area of cortical distribution lie the motor and sensory areas for the opposite half of the body, excluding leg, foot and perineum (which are in anterior cerebral territory), and the auditory and speech areas.
The anterior cerebral artery leaves the internal carotid artery at the anterior perforated substance and passes forwards above the optic nerve (Fig. 7.19). It is connected to its fellow of the opposite side by the anterior communicating artery. It is distributed to the orbital surface of the frontal lobe and to the whole of the medial surface of the hemisphere above the corpus callosum as far back as the parieto-occipital sulcus (see Figs 6.106 and 6.107, p. 449). Its distribution extends over the superior border to meet the area supplied by the middle cerebral artery (Figs 7.13 and 7.14). The motor and sensory areas for the opposite leg, foot and perineum, including the micturition and defecation centres, lie in its territory. Because of the anastomosis via the anterior communicating artery, it is usually possible for one anterior cerebral to be supplied with blood from the contralateral internal carotid. Very occasionally both anterior cerebrals may arise from one carotid by a common stem.
The fact that the internal carotid gives origin to the anterior and middle cerebral arteries (supplying the sensorimotor cortex and internal capsule; see below) and to the ophthalmic artery (supplying the retina, see p. 403) accounts for the characteristic combination of blindness in one eye with contralateral hemiplegia that may follow stenosis or occlusion of the internal carotid artery. However, occlusion may be ‘silent’ because of collateral circulation through the arterial circle; the effects are very variable, depending on the state and size of the vessels.
The posterior cerebral artery curls back around the cerebral peduncle (Fig. 7.19), supplying it and the optic tract, and passes back above the tentorium to supply the inferomedial surface of the temporal and occipital lobes (Figs 7.13 and 7.14). Its territory meets that of the anterior cerebral artery at the parieto-occipital sulcus. Its branches extend around the borders of the brain to supply the inferior temporal gyrus and a corresponding strip of cortex on the lateral surface of the occipital lobe. The visual area for the opposite field of vision lies wholly within its territory, but the middle cerebral branches can sometimes extend sufficiently far back on the occipital lobe to supply the macular part of the visual area (see p. 464). Thus the macular field of vision may be spared when the rest of the visual area is destroyed by a posterior cerebral thrombosis. The posterior cerebral may receive some or all of its blood from the internal carotid and not the basilar; indeed, this is the primitive embryonic condition where the posterior cerebral is a branch of the carotid. The basilar system is a later development which joins the original posterior cerebral, whose proximal end becomes the posterior communicating. The two posterior communicating arteries are often unequal in size.
The arterial supply of the subcortical nuclei is by branches from the three cerebral vessels, and by the anterior choroidal. Branches from the cerebral vessels enter through the perforated substances.
The anterior perforated substance (Fig. 7.18) receives numerous small branches mainly from the commencement of the middle cerebral artery. These are the striate branches and they supply the internal capsule, as well as the thalamus and basal nuclei. One of the lateral striate, or lenticulostriate, arteries, usually the largest, is Charcot’s ‘artery of cerebral haemorrhage’ whose rupture or occlu-sion are the most common causes of a typical ‘stroke’ with contralateral hemiplegia. The medial striate artery is usually a branch of the anterior cerebral artery.
Branches from the posterior cerebral artery enter the posterior perforated substance to reach the thalamus and basal ganglia, penetrating the posterior part of the internal capsule on the way.
The anterior choroidal artery supplies the choroid plexus, passing below the optic tract to enter the inferior extremity of the choroid fissure at the tip of the inferior horn of the lateral ventricle, just above the uncus. The artery gives branches to the optic tract and radiation and the lateral geniculate body, as well as to the posterior part of the internal capsule, basal nuclei and limbic system. The choroid plexus also receives a few additional twigs from the posterior cerebral artery, which enter the choroid fissure behind the thalamus.
Effects of arterial occlusion. Obviously the effect of occlusion of the cerebral arteries will vary with the degree and site of obstruction, but the main effects of complete occlusion may be summarized as follows.
Anterior cerebral: contralateral weakness and sensory loss (mainly leg, foot and perineum).
Middle cerebral: contralateral hemiplegia and hemianaesthesia (with aphasia if the lesion is left-sided).
Posterior cerebral: contralateral hemianopia and hemianaesthesia.
Anterior choroidal: contralateral hemianopia and hemianaesthesia, with some degree of hemiplegia.
Cerebral veins
The venous return does not follow the arterial pattern. Unlike the cortical arteries, which tend to travel deep in the sulci, the cortical veins tend to travel superficially, adherent to the deep surface of the arachnoid mater that bridges each sulcus. They then usually cross the subdural space to drain into the nearest available venous sinus of the dura mater, generally entering obliquely against the bloodstream.
The superolateral and superomedial surfaces of the hemisphere drain into the superior sagittal sinus (Fig. 7.15) by several superior cerebral veins. A few inferior cerebral veins drain into the transverse sinus. In each case the veins mainly enter against the direction of bloodflow. The superior veins, if encountering a blood lake, pass on its cerebral surface beneath the arachnoid (the blood lakes are between the ‘two layers’ of the dura; Fig. 7.16).