CHAPTER 12 Cell populations at the start of organogenesis
SPECIFICATION OF THE BODY AXES AND THE BODY PLAN
Axes may be conferred on the whole embryonic disc, which is initially flat and mainly two-dimensional. However, their subsequent orientation in the folded three-dimensional embryo will be completely different. The dorsal structures of the folded embryo form from a circumscribed central ellipse of the early flat embryonic disc (see Fig. 10.5). Lateral and ventral structures form from the remainder of the disc, and the peripheral edge of the disc eventually becomes constricted at the umbilicus (see Figs 10.9 and 10.10). Although the appearance of part of the epiblast is taken to specify the dorsal surface of the embryo, the inner layer, i.e. the hypoblast, is not by default a ventral embryonic structure.
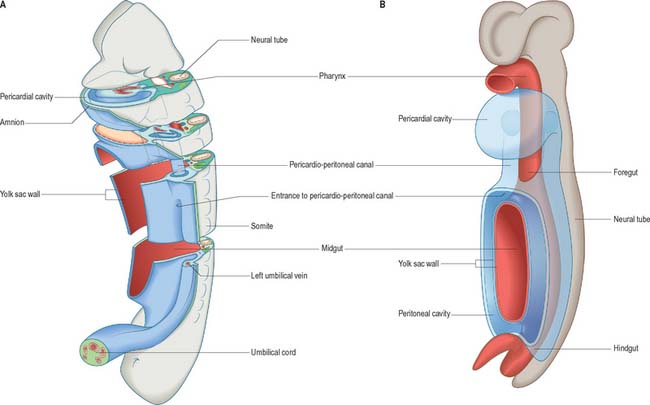
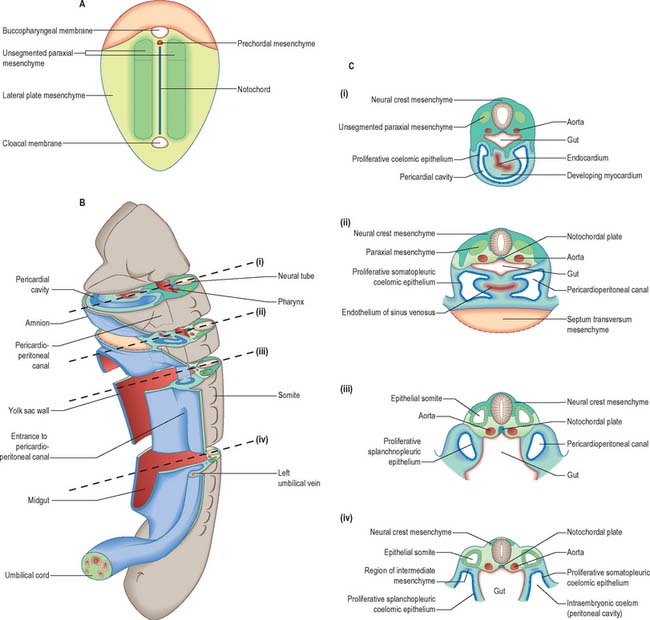
The development of all the body organs and systems, organogenesis, begins after the dramatic events of gastrulation, when the embryo has attained a recognizable body plan. In human embryos this corresponds to the end of stage 10 (Fig. 12.1). The head and tail folds are well formed, with enclosure of the foregut and hindgut (proenteron and metenteron), although the midgut (mesenteron) is only partly constricted from the yolk sac. The forebrain projection dominates the cranial end of the embryo, and the buccopharyngeal membrane and cardiac prominence are caudal and ventral to it. The cardiac prominence contains the transmedian pericardial cavity, which communicates dorsocaudally with right and left pericardioperitoneal canals. These pass dorsally to the transverse septum mesenchyme and open caudally into the extraembryonic coelom on each side of the midgut. The intraembryonic mesenchyme has begun to differentiate and the paraxial mesenchyme is being segmented into somites. Neural groove closure is progressing caudally, so that a neural tube is forming between the newly segmenting somites. Rostrally, the early brain regions, which have not yet fused, can be discerned. The neuroepithelium is separated from the dorsal aspect of the gut by the notochord. The earliest blood vessels have appeared, and a primitive tubular heart occupies the pericardium. The chorionic circulation is soon to be established, after which the embryo rapidly becomes completely dependent upon the maternal bloodstream for its requirements. The embryo is connected to the developing placenta by a mesenchymal connecting stalk in which the umbilical vessels develop, and which also contains the allantois, a hindgut diverticulum. The lateral body walls are still widely separated. The embryo has contact with three different vesicles: the amnion, which is in contact with the surface ectoderm; the yolk sac, which is in contact with the endoderm; and the chorionic cavity, containing the extraembryonic coelom, which is in contact with the intraembryonic coelomic lining (see Fig. 10.10).
The degree to which vertebrate embryos are developmentally constrained at this period of development is controversial. Comparative studies on the timing at which specific embryonic structures appear, heterochrony, have shown that other embryonic species do not follow the same developmental sequence as humans (Richardson & Keuck 2002). Although some developmental mechanisms are highly conserved, e.g. the homeobox gene codes, others may have been dissociated and modified in different vertebrate species during evolution.
EMBRYONIC CELL POPULATIONS AT THE START OF ORGANOGENESIS
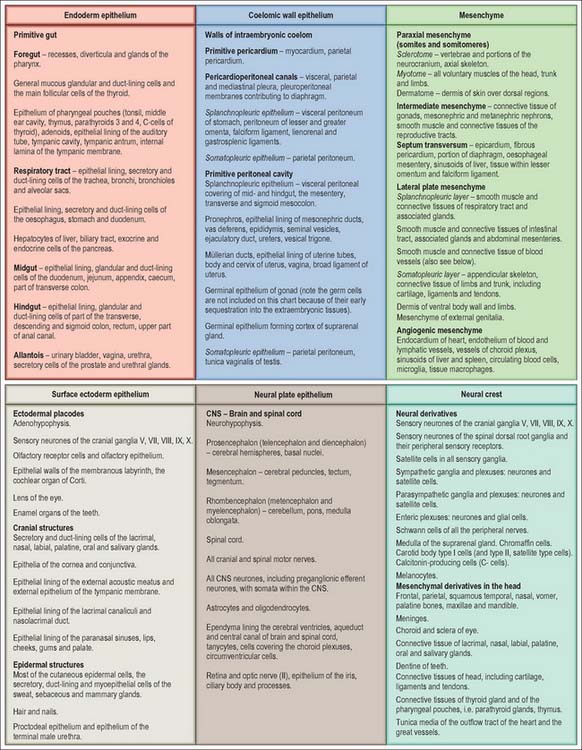
The developmental processes operating in the embryo between stages 5 and 9 enabled the construction of the bi- and tri-laminar embryonic disc, the intraembryonic coelom and new proliferative epithelia. From the end of stage 10, a range of local epithelial and mesenchymal populations now interact to produce viscera and appendages. The inductive influences on these tissues and their repertoire of responses are very different from those seen at the onset of gastrulation. The range of tissues present at the start of organogenesis, when the body plan is clear, is given below and shown in Figs 12.1 and 12.2. For a summary of the fates of the embryonic cell populations, see Fig. 12.3.