chapter 16 Catabolism of macromolecules and energy generation
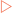 Reduced cofactors pass electrons to the electron transport chain, which generates a proton gradient.
Reduced cofactors pass electrons to the electron transport chain, which generates a proton gradient.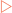 Fatty acids are broken down in the mitochondrion by the β-oxidation pathway giving acetyl CoA and reduced cofactors.
Fatty acids are broken down in the mitochondrion by the β-oxidation pathway giving acetyl CoA and reduced cofactors.Catabolism
Specific pathways for each of the macronutrients are discussed as is the way in which these pathways all converge and use a common mechanism to generate the majority of ATP (Fig 16-1).
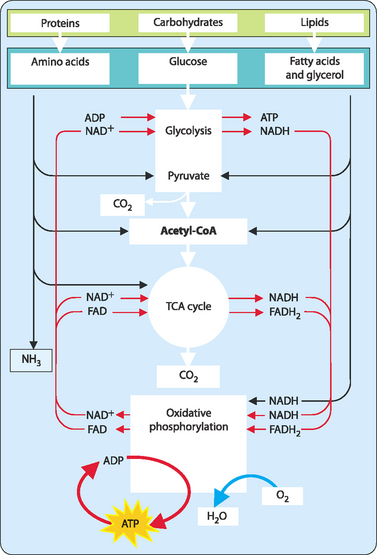
FIGURE 16-1 An overview of the main catabolic pathways of metabolism.
[Based on Baynes & Dominiczak, 2009, Medical Biochemistry, 3rd edition, Mosby]
Glycolysis
Glycolysis is a branching metabolic pathway that occurs in the cytoplasm of eukaryotic cells. It consists of 10 enzymes that combine to convert the 6-carbon glucose molecule into two molecules of the 3-carbon compound pyruvate (Fig 16-2)
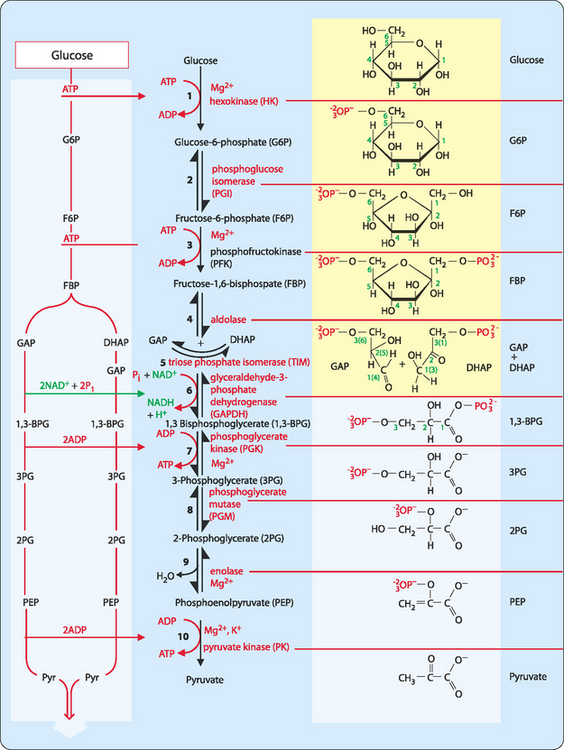
FIGURE 16-2 The glycolytic pathway.
[Based on Baynes & Dominiczak, 2009, Medical Biochemistry, 3rd edition, Mosby]
The investment stage
Finally, the 6-carbon FBP is split by the enzyme aldolase into two 3-carbon molecules, glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP). However, DHAP is not a substrate for the next enzyme in the pathway, GAP dehydrogenase (GAPDH). The enzyme triose phosphate isomerase is able to interconvert GAP and DHAP. As GAP is being consumed by the rest of the glycolytic pathway this drives the conversion of DHAP to GAP.
Making an ATP profit
As can be seen in Figure 16-2 all further steps of glycolysis occur twice for each glucose molecule that enters the pathway. In this stage of glycolysis there are three key reactions that ensure the creation of more ATP than the initial investment made in the early stages.
Controlling glycolysis
As with any metabolic pathway, glycolysis needs to be controlled so that it responds to changes in the physiological environment of the cell. It is of particular importance in this case as some of the enzymes of glycolysis are shared by the competing pathway gluconeogenesis (see Ch 17).
As was seen in Chapter 14 the activity of just one enzyme in a pathway can affect flux through that pathway, and that the controlling enzymes are those that catalyse the non-equilibrium, irreversible reactions. In glycolysis the enzymes HK, PFK and PK all catalyse irreversible reactions, are unique to glycolysis and are control points. The control of glycolysis in humans occurs at three levels: at the level of the enzyme, at the level of the cell and at the level of the body.
At the level of the cell it is the enzyme PFK that is the key to control. ATP, PEP and citrate inhibit PFK, whereas ADP and AMP stimulate it (Fig 16-3). This allows PFK to respond to a number of things:
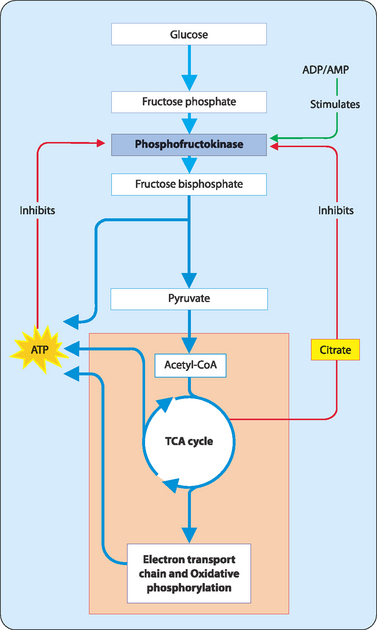
FIGURE 16-3 Phosphofructokinase is a key control point for glycolysis.
[Based on Campbell & Reece, 2005, Biology, 7th edition, Pearson Benjamin Cummings]