Carotid Body Tumors
Wesley S. Moore
Introduction
The only known pathologic condition associated with the carotid body is a carotid body tumor. The carotid body was first described by Von Haller in 1743 and Heller coined the term glomus tumor in 1762. In spite of the long history, these lesions, even today, remain a topic surrounded by considerable misunderstanding and confusion. Much of this misconception can undoubtedly be traced to the lack of established data related to the histologic origin, biologic behavior, and incidence of malignancy associated with carotid body tumors. As one might predict, this situation has generated considerable controversy regarding the appropriate management of these lesions.
Carotid body tumors are uncommon neoplasms. Their exact incidence remains unknown. In 1971, <500 cases had been recorded in the world literature, and by the early 1980s, this figure had doubled to just >1,000. Like most other types of benign and malignant neoplasms, the precise cause of carotid body tumors is not known. Also, as is frequently the case with other tumors that have the potential for endocrine activity, these lesions are recognized to occur in three distinct forms: sporadic, familial, and hyperplastic. The sporadic form is more common, although the familial pattern has been recognized in as many as 10% of cases by some authors. A review of familial cases of cervical paragangliomas suggests an autosomal dominant inheritance pattern. Hyperplastic lesions are associated with hypoxia and include patients with chronic obstructive pulmonary disease (COPD), congenital heart disease, and those living at high altitude. It has long been appreciated that chronic hypoxia is associated with enlargement of the carotid body in some patients. Although these changes are usually not classified as tumors, histologic examination of carotid body tissue from these patients frequently reveals marked cellular hyperplasia. This observation, combined with epidemiologic data that suggest an increased incidence of carotid body neoplasms occurring in individuals who live at high elevations, has led some investigators to implicate prolonged hypoxemia as a possible causal factor related to the development of these uncommon tumors.
This chapter reviews my current practice for the treatment of patients who have
carotid body tumors. Because many of these management principles are based on an understanding of the pathologic and biologic behavior of these lesions, I have included a summary of the current understanding of these important topics. With an appreciation of these principles and the use of operative techniques developed from them, one should be able to safely manage even the most challenging carotid body lesions. Alternatively, inadequately planned neck explorations in improperly prepared patients or biopsies of seemingly unimpressive neck masses often result in disastrous consequences. Even when the presence of a carotid bifurcation paraganglioma is correctly diagnosed preoperatively, resection of this lesion can be a humbling experience even for the most experienced surgeons.
carotid body tumors. Because many of these management principles are based on an understanding of the pathologic and biologic behavior of these lesions, I have included a summary of the current understanding of these important topics. With an appreciation of these principles and the use of operative techniques developed from them, one should be able to safely manage even the most challenging carotid body lesions. Alternatively, inadequately planned neck explorations in improperly prepared patients or biopsies of seemingly unimpressive neck masses often result in disastrous consequences. Even when the presence of a carotid bifurcation paraganglioma is correctly diagnosed preoperatively, resection of this lesion can be a humbling experience even for the most experienced surgeons.
Pathology
The normal carotid body is an ovoid nodule, measuring approximately 0.5 × 0.5 cm, located along the posterior surface of the carotid bifurcation. This structure represents the largest mass of chemoreceptive tissue found anywhere in the body. The blood supply to the carotid body is usually derived from branches of the external carotid artery, and the venous return occurs through tributaries of the lingual and laryngopharyngeal veins. The innervation is exclusively sensory. Small nerve fibers arising from the carotid body join the larger glossopharyngeal nerve before entering the cranium through the jugular foramen. Stimuli that evoke a response by the chemoreceptor cells include increases in the plasma carbon dioxide tension or blood temperature, or a decrease in the plasma pH or arterial oxygen tension. Once activated, the stimulation of carotid body chemoreceptors causes several physiologic responses, including increased minute volume ventilation, increased pulse rate, and an elevation of the mean systemic blood pressure. Embryologically, the carotid body derives elements from both neural crest ectoderm and mesodermal tissue originating from the third branchial arch. The neural crest ectoderm differentiates into chemoreceptor cells that migrate in close association with the autonomic ganglion cells, and are therefore frequently referred to as paraganglioma cells. The mesodermal tissue develops into a highly vascular fibrous stroma that supports and nurtures these clusters of chemoreceptor cells.
Carotid body tumors are neoplastic growths that arise from these chemoreceptive structures. The morphologic appearance of carotid body tumors closely resembles that of normal carotid body tissue. Grossly, the tumor is reddish brown and highly vascular. Microscopically, one sees chemoreceptor cells embedded in a fibrous stroma situated adjacent to abundant capillaries. Overall, the lesions have a very well-differentiated benign appearance. Degenerative malignant characteristics, such as unusual nuclear forms, increased mitotic activity, and vascular invasion, are only very rarely seen. Historically, there has been considerable confusion regarding the terminology and classification of these tumors. The term glomus tumor is a misnomer and was introduced based on the mistaken impression that these highly vascular tumors were in fact a form of neurovascular arteriovenous malformation. Mulligan introduced the term chemodectoma to describe these lesions in 1950, because these tumors are composed of cells with both chemoreceptor and secretory functions. Advanced immunohistochemical studies have subsequently suggested that carotid body tumors are capable of synthesizing numerous different neuroendocrine substances. Secretory granules containing a wide variety of neuropeptides and several enzymes involved in the cytoplasmic synthesis of catecholamines have all been demonstrated. For unclear reasons, however, patients who have carotid body tumors almost never manifest any clinical evidence of excessive endocrine hormone secretion. Sporadic case reports have noted secretory activity of norepinephrine and dopamine, but these are the exceptions. Because it is believed that these tumors arise embryologically from epithelioid cells of the neural crest that have migrated with neural tissue adjacent to the autonomic ganglion, they have also been referred to as paragangliomas. Paragangliomas actually comprise a family of neoplastic tumors that can occur anywhere along the autonomic ganglion chain, from the organ of Zuckerkandl to the glomus intravagale. Classically, paragangliomas arising in the adrenal medulla secrete high levels of catecholamines and usually stain positive for chromaffin. Despite having been found to contain granules rich in catecholamines within their cytoplasm, carotid body tumors are most commonly nonfunctional and classically stain negative for chromaffin.
Grossly, these tumors are highly vascular and often invade the adventitia of the adjacent carotid vessels. The tumors grow slowly, beginning along the posterior aspect of the carotid bifurcation. As they enlarge, they typically gradually widen the angle between the internal and the external carotid arteries. Frequently, when carotid body tumors grow very large, they encase the main trunk and proximal tributaries of the external carotid artery. Without explanation, however, they only rarely do the same to the internal carotid vessel. This phenomenon may be related to the origin of the blood supply to the tumor from branches of the external carotid artery. Nonetheless, it is a consistent feature found in large carotid body tumors and has important ramifications related to the strategy for resection, which is discussed in more detail later in section on “Resection of the Tumor.”
Although most carotid body tumors are considered benign lesions, malignancy does occur. It is generally accepted that the biologic behavior of a given carotid body tumor cannot be predicted based solely on the histologic appearance of the resected specimen. Benign-appearing tumors are capable of both locally aggressive growth and nodal and distant metastatic spread. Adherence to vascular and neurologic structures is well known, and even extension through the base of the skull into the cranium has been described. Therefore, as is the case with many other neuroendocrine tumors, malignancy is determined by how the lesion behaves clinically. This is quite different from most other types of cancers, in which histologic appearance remains the primary criterion for identification of malignancy. If enlarged lymph nodes are encountered during dissection, they should be removed for biopsy. The presence of carotid body cells within an adjacent lymph node is diagnostic of malignancy.
By far, the most common presentation of a carotid body tumor is that of an incidentally noted asymptomatic neck mass. The lesion typically causes no pain and is located just below the angle of the mandible. Usually, the tumors grow slowly over several years; however, more rapidly growing lesions are occasionally encountered. In a review by Patetsios et al. from Baylor University in Dallas, they found that 62% of their cases presented with a painless neck mass, 10% with a painful mass, and a few with cranial nerve deficits. Most carotid body tumors are diagnosed in the third or fourth decade of life, but they have been reported in younger and older patients. No gender predominance has yet been established. When the tumor occurs in a familial pattern of inheritance, there is up to a 30% incidence of bilateral tumors, as opposed to 2% to 20% in nonfamilial cases.
Occasionally, when a carotid body tumor becomes very large, compression or local invasion of adjacent structures can lead to a variety of different symptoms. Local discomfort such as pain, fullness, and numbness is not uncommon. In this situation, some patients may complain of difficulty in swallowing, hoarseness, and chronic cough due to compression of the airway or involvement of adjacent cranial nerves.
Despite the fact that carotid body tumors are frequently classified as neuroendocrine neoplasms, clinical evidence of any endocrine imbalance resulting from secretory function of these lesions is almost never encountered. Interestingly, however, the histologic machinery to do so is apparently present. As was mentioned previously in section on “Pathology,” careful ultrastructural and immunohistochemical examination of these tumors has revealed that carotid body tumors are capable of producing secretory granules containing a large variety of catecholamines. Nevertheless, “functional” cervical paragangliomas are exceedingly rare. Only very rarely is a case reported describing a patient who has both hypertension and a concomitant neck mass, and in whom the hypertension resolves after resection of the associated carotid body tumor. The assumption, although not proven, is that the hypertension in these cases occurs on the basis of excessive “tumor-related” catecholamine secretion.
On examination, these lesions are often described as painless fixed masses located at the angle of the jaw. In fact, more careful examination usually demonstrates that, although movement is limited longitudinally, the tumor can readily be moved in a lateral direction. This is an important feature of the physical examination and helps establish an association of the mass with the longitudinally running neurovascular structures. Typically, the mass is firm, smooth, and lobulated. Frequently, transmission of the carotid pulsation is appreciated during palpation. Furthermore, in approximately 30% to 40% of patients, an audible bruit is present over the tumor, giving testimony to the highly vascular nature of the tumor. Locally aggressive tumors may invade or compress adjacent cranial nerves. In fact, careful examination yields clinical evidence of nerve dysfunction in <10% of patients who present with carotid body tumors. Most commonly, the hypoglossal or vagal nerves are affected, but laryngeal nerve involvement and Horner syndrome have also occasionally been reported. Large tumors can extend into the base of the skull or present as a bulge in the lateral wall of the oropharynx with deviation of the soft palate. Ischemic cerebrovascular symptoms rarely, if ever, occur. Patients who have carotid body tumors who also present with transient or permanent ischemic events are usually found to have concomitant carotid bifurcation atheroma or another unrelated cause to account for the cerebrovascular systems.
The differential diagnosis of a patient who presents with an enlarging neck mass is extensive. Congenital lesions, such as branchial cleft abnormalities, hygromas, and vascular malformations, should all be considered. Chronic lymphadenitis, reactive lymphadenopathy, and other inflammatory reactions must also be excluded. Many other benign and malignant neoplastic processes, such as lipomas, neurofibromas, salivary gland and parotid tumors, metastatic head and neck cancers, leukemias, and lymphomas, can also present in this way. It should also be remembered that other cervical paragangliomas exist. These include the glomus vagale, and the glomus jugulare. It is beyond the scope of this chapter to describe their management other than to mention that it may be quite different from the management of carotid body tumors. Vascular abnormalities, such as carotid aneurysms, coils, and kinks, should also not be overlooked. A thorough history and physical examination combined with carefully selected diagnostic studies can usually distinguish these other pathologies and successfully identify most cervical paragangliomas. Clearly, however, this orderly workup is critical, as the appropriate treatment for many of these lesions varies greatly.
Once identified, all carotid body tumors should be removed. Although these lesions are slow-growing neoplasms with a relatively low risk of malignancy, small tumors are easier to excise, and eventually most, if not all, will progress and become locally invasive. These tumors continue to grow and, in time, extension to the base of the skull or involvement of cranial nerves may complicate their management. In addition, the threat of distant metastases, although low, is real. Despite being categorized as generally benign, malignant transformation of carotid body tumors has been reported. The exact incidence of malignancy is difficult to quantitate and depends on the definition of the term used. The biologic behavior and malignant potential of a given carotid body tumor cannot be predicted by histologic examination. Rather, malignant potential is identified by clinical behavior. If strict criteria of regional lymph node or distant metastatic spread were used, the incidence of metastatic spread from carotid body tumors would be quite low, although it is definitely not zero. Some reports have suggested that the true incidence of distant metastases in untreated lesions approaches 10%. However, locally aggressive growth patterns of carotid body tumors are not at all uncommon, and certainly lesions that invade adjacent structures can be considered at least potentially malignant. Consequently, most authorities consider all cervical paragangliomas capable of malignant behavior. Death from carotid body tumors does occur, and not always from metastatic disease. Relentless local growth of these lesions can cause death by airway compression, cranial neuropathies, or intracranial extension. Also, regional lymph node and distant metastases, which are exceedingly rare at presentation, can occur years after the primary tumor has been resected. It should also be mentioned that, because of this low risk of malignancy, a case could be made against operative treatment in the high-risk or elderly surgical patient who presents with a small, asymptomatic carotid body tumor. In most cases, however, in view of the natural history of progressive enlargement, frequent local invasion, and small but unpredictable risk of distant metastases, most surgeons recommend surgical resection once the diagnosis of a carotid body tumor has been established.
The preoperative evaluation of patients being considered for resection of a carotid body tumor is important and should be directed to address several questions:
What is the overall general medical condition of the patient?
Are other cervical paragangliomas present?
What is the anatomic extent of the lesion under investigation?
Is there any cranial nerve involvement detectable preoperatively?
Is there any evidence of endocrine dysfunction or hormonal imbalance?
Each patient should undergo a thorough history, and important risk factors should be identified. Likewise, the physical examination should be complete, with particular emphasis on the neurologic and
cerebrovascular systems. Clearly, an asymptomatic elderly patient who is considered to be a poor operative risk may not be well served by a potentially hazardous resection of what is most commonly a benign neoplasm. Likewise, the presence of multiple lesions or extension of a tumor into the base of the skull would obviously influence the operative approach. Involvement of cranial nerves suggests at least a locally aggressive tumor, and documentation of these deficits preoperatively is obviously very important. Several imaging techniques are now available to diagnose and characterize carotid body tumors. As is frequently the case, each of these modalities has specific strengths and weaknesses that should be carefully considered.
cerebrovascular systems. Clearly, an asymptomatic elderly patient who is considered to be a poor operative risk may not be well served by a potentially hazardous resection of what is most commonly a benign neoplasm. Likewise, the presence of multiple lesions or extension of a tumor into the base of the skull would obviously influence the operative approach. Involvement of cranial nerves suggests at least a locally aggressive tumor, and documentation of these deficits preoperatively is obviously very important. Several imaging techniques are now available to diagnose and characterize carotid body tumors. As is frequently the case, each of these modalities has specific strengths and weaknesses that should be carefully considered.
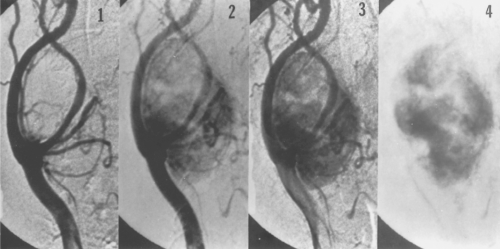
The preoperative evaluation of patients who have carotid body tumors has traditionally relied on the results of selective carotid angiography. This is largely due to the unmatched demonstration of anatomic detail in a familiar format that is easily interpreted by most clinicians. The carotid bifurcation is particularly easy to demonstrate with this examination. Information regarding the overall size, proximal and distal extent, and degree of vascularity of the lesion can usually be easily assessed (Fig. 1). Information regarding the major arterial blood supply to the tumor is also obtained. Infrequently, an unusual tumor blood supply from an aberrant ascending cervical or vertebral artery branch is identified, which can be very important preoperatively. Another advantage of angiography is its ability to evaluate the entire distribution of the brachiocephalic and cerebrovascular tree, including the origin of the vessels as they arise from the aortic arch and the intracranial portion of the branch vessels. This is important because angiography is an extremely sensitive method of identifying cervical paragangliomas and allows the surgeon to easily detect occult nonpalpable synchronous or contra lateral tumors. Also, angiography can occasionally suggest malignancy by demonstrating encasement of the carotid vessels. Rarely, this may alert the surgeon to the presence of extensive internal carotid involvement and allow preoperative planning for the possible need for a vascular reconstruction. Last, by imaging the lumen of the carotid vessels, any atheromatous plaques or significant ulcerations can be documented. Kinks, coils, aneurysms, and any other unsuspected anatomic variations are also all easily identified with this technique. Intracranial pathology that may influence management decisions includes arteriovenous malformations, cerebral aneurysms, and significant siphon stenoses. Finally, some authors have reported the use of preoperative embolization as an adjunct before the resection of particularly large or highly vascular carotid body tumors. As such, although I have not found this necessary (and consider it potentially hazardous), one might qualify the ability to consider this treatment option as another potential advantage to support the use of angiography during the preoperative evaluation of these patients.
The risks associated with cerebral angiography are numerous. First, this technique is an invasive procedure that requires arterial access, and wound complications are therefore not infrequent. Hematoma formation, acute arterial dissection, false aneurysms, and distal embolization have all been reported. The incidence of these complications has not been documented consistently; however, they probably occur in between 1% and 2% of cases. Second, catheter manipulation within the carotid artery can lead to serious complications as a result of dissection or embolization. In one review of over 5,000 cerebral angiographic procedures, the overall risk of neurologic sequelae was 0.9%, with 0.06% of patients experiencing a permanent stroke. Others have placed the risk of neurologic events and stroke at 2.6% and 0.6%, respectively. Although most of these reviews include patients being evaluated for atherosclerotic vascular disease and not carotid body tumors, it is probably accurate to
estimate that, during cerebral angiography, the risk of a neurologic event is, 1%. Finally, the contrast injected during angiography can induce allergic reactions, renal failure, and alterations in the coagulation system. Allergic reactions range from mild rashes and gastrointestinal upset to life-threatening hypotension, bronchospasm, pulmonary edema, and cardiac arrhythmias. The risk of contrast reactions in all types of angiographic studies varies between 2% and 8%. For cerebral examinations, risk is in the lower range, with a rate of, 2%. In high-risk patients who have antecedent renal disease, the risk of acute renal failure after the administration of contrast can be as high as 40%, with >8% of patients requiring permanent dialysis. Finally, depending on the institution, the cost of angiography is as high as $5,000 per procedure.
estimate that, during cerebral angiography, the risk of a neurologic event is, 1%. Finally, the contrast injected during angiography can induce allergic reactions, renal failure, and alterations in the coagulation system. Allergic reactions range from mild rashes and gastrointestinal upset to life-threatening hypotension, bronchospasm, pulmonary edema, and cardiac arrhythmias. The risk of contrast reactions in all types of angiographic studies varies between 2% and 8%. For cerebral examinations, risk is in the lower range, with a rate of, 2%. In high-risk patients who have antecedent renal disease, the risk of acute renal failure after the administration of contrast can be as high as 40%, with >8% of patients requiring permanent dialysis. Finally, depending on the institution, the cost of angiography is as high as $5,000 per procedure.
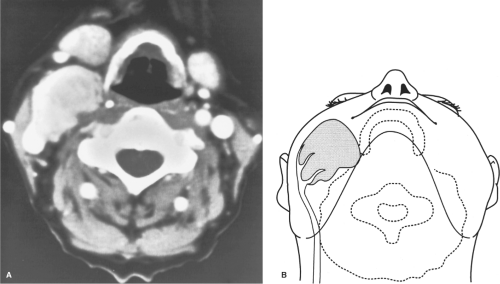 Fig. 2. A: CT scan demonstrating large carotid body tumor with probable encasement of the external carotid artery. B: This is the artist’s concept of the relationship of the carotid body tumor to the carotid artery, as seen in Figure 2A.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|