HF, heart failure; ECG, electrocardiogram; MI, myocardial infarction; TIA, transient ischemic attack.Data from Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–1049.
TABLE 2-2 American Society of Anesthesiologists Physical Status Classification

TABLE 2-3 Estimated Metabolic Equivalents (METs) of Various Activities
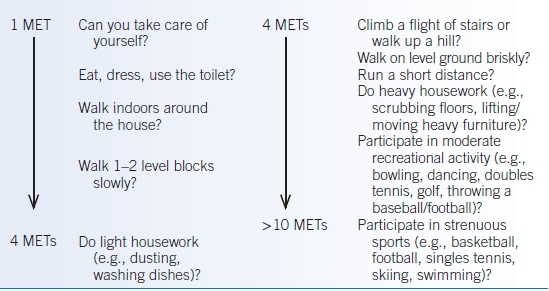
Data from Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–654; Fletcher GF, Balady G, Froelicher VF, et al. Exercise standards: statement for healthcare professionals from the American Heart Association. Circulation 1992;86:340–344; Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. Circulation 2007;116:e418–e499.
Physical Examination
- A complete physical examination is essential.
- Specific attention should be paid to the following:
- Vital signs, particularly blood pressure (BP). Systolic blood pressure (SBP) of <180 mm Hg and diastolic blood pressure (DBP) of <110 mm Hg are generally considered acceptable. The management of SBP >180 mm Hg or DBP >110 mm Hg is controversial. Postponing elective surgery to allow adequate BP control in this setting is reasonable but poorly studied. The appropriate length of time to wait after treatment is instituted is also unclear.
- Murmurs suggestive of significant valvular stenosis or regurgitation.
- Evidence of heart failure (HF) (jugular venous distension, crackles, S3 gallop, peripheral edema).
- Vital signs, particularly blood pressure (BP). Systolic blood pressure (SBP) of <180 mm Hg and diastolic blood pressure (DBP) of <110 mm Hg are generally considered acceptable. The management of SBP >180 mm Hg or DBP >110 mm Hg is controversial. Postponing elective surgery to allow adequate BP control in this setting is reasonable but poorly studied. The appropriate length of time to wait after treatment is instituted is also unclear.
- A thorough preoperative neurologic examination may identify patients with baseline cognitive impairment or dementia who are at increased risk for delirium, and serves as a useful comparison if there is a question of altered mental status perioperatively.
Diagnostic Criteria
Figure 2-1 presents an overview of the 2014 ACC/AHA guidelines on perioperative cardiovascular assessment and management of patients undergoing noncardiac surgery.1
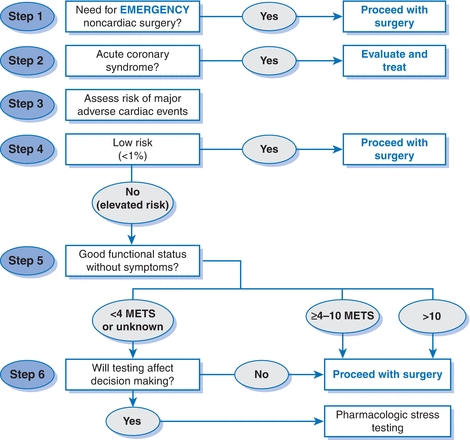
Figure 2-1 ACC/AHA cardiovascular risk assessment algorithm. Modified from Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014;64:e77–e137.
Step 1
- Determine the urgency of surgery—emergent or not.
- If truly emergent, proceed to surgery.
- Perioperative monitoring and management should be based on the presence of or clinical risk factors for CAD.
Step 2
- For urgent or elective surgery, determine if an ACS is present.
- If the patient has an ACS, postpone surgery, evaluate, and treat based on guideline-directed medical therapy (GDMT).
Step 3
Estimate perioperative risk of a MACE based on combined clinical/surgical risk. This estimate can be generated by incorporating the RCRI with an estimation of surgical risk or by using the NSQIP risk calculator (http://www.surgicalriskcalculator.com (accessed 1/19/15).17,18
Step 4
If the patient has a low risk of a MACE (<1%), no additional testing is required and the patient may proceed to surgery.
Step 5
- If the patient is at elevated risk of a MACE (≥1%), determine functional capacity by either patient report or objective measure such as the DASI.45–47
- If the patient has a functional capacity of ≥4 METs without symptoms, then proceed to surgery without further evaluation.
Step 6
- A patient with poor (<4 METs) or unknown functional capacity may need additional testing, but only if it will impact patient decision making (i.e., willingness to undergo percutaneous coronary intervention [PCI] or CABG depending on the results of the test) or perioperative care.
- Stress testing is appropriate in this context. If the stress test is abnormal, coronary angiography and revascularization should be pursued. Following this, the patient can either proceed to surgery or consider alternative strategies, such as noninvasive treatment or palliative care. If the stress test is normal, proceed to surgery.
Step 7
If testing will not affect decision making or perioperative care, then proceed to surgery with optimal medical management or consider alternate strategies as above.
Diagnostic Testing
12-Lead Electrocardiogram
- The current ACC/AHA guidelines recommend a preoperative resting 12-lead ECG for patients with known CHD, significant arrhythmia, PAD, cerebrovascular disease, or structural heart disease, except for those undergoing low-risk surgery.1
- In patients with established CHD, the baseline ECG contains prognostic information relating to short- and long-term morbidity and mortality.
- Although no data exist regarding the optimal time interval between obtaining an ECG and elective surgery, general consensus suggests 1 to 3 months is adequate.
- In patients with established CHD, the baseline ECG contains prognostic information relating to short- and long-term morbidity and mortality.
- Preoperative resting ECG may also be considered for asymptomatic patients without known CHD, except for those undergoing low-risk surgery.
- The primary utility of a preoperative ECG in most patients is for comparison with ECGs that may be obtained postoperatively. Therefore, the value of the baseline ECG increases with the risk of the surgical procedure.
Resting Echocardiogram
- Several studies have demonstrated an association between reduced left ventricular (LV) systolic function and perioperative complications, particularly postoperative HF, with the greatest risk in patients with an left ventricular ejection fraction (LVEF) of <35%. Assessment of EF by echocardiogram has relatively high specificity for predicting perioperative cardiac events, but only modest incremental predictive power over clinical risk factors.48
- Indications for preoperative echocardiography are no different than in the nonoperative setting, and is not routinely recommended.1
- Auscultated murmurs suggestive of significant underlying valvular disease should be evaluated by echocardiography, as should occur in a nonoperative setting. Of significant note, the surgical risk of asymptomatic severe aortic stenosis is no longer considered prohibitively high as to be an absolute contraindication to elective noncardiac surgery.1
- The most recent ACC/AHA guidelines recommend preoperative evaluation of LV function with resting echocardiogram for the following1:
- Patients with dyspnea of unknown origin.
- Patients with known HF and worsening dyspnea or other change in clinical status.
- In patients with a prior history of LV dysfunction, reassessment of LV function may be considered if more than a year has elapsed since the last evaluation.
- Patients with dyspnea of unknown origin.
Noninvasive Stress Testing
- Routine stress testing of all patients undergoing surgery is not warranted. The decision to pursue a stress evaluation should be guided by assessment of perioperative risk and functional capacity, as detailed above.
- Importantly, stress testing should not be performed unless the patient is willing to undertake subsequent clearly indicated treatments.
- Noninvasive stress testing is not routinely indicated for patients at low risk for noncardiac surgery.
- The 2014 ACC/AHA guidelines additionally recommend the following1:
- Noninvasive stress testing (exercise or pharmacologic) for patients who are at an elevated risk of MACE and have either poor (<4 METs) or unknown functional capacity, if it will change management.
- For patients with elevated risk and excellent (>10 METs) functional capacity, it is reasonable to forgo further exercise testing and proceed to surgery.
- Consider noninvasive stress testing for patients with elevated risk and moderate (4 to 6 METs) or good (7 to 10 METs) functional capacity, only if it will alter perioperative care.
- Noninvasive stress testing (exercise or pharmacologic) for patients who are at an elevated risk of MACE and have either poor (<4 METs) or unknown functional capacity, if it will change management.
Coronary Angiography
- Routine preoperative coronary angiography in all patients is not recommended.1
- The indications for preoperative coronary angiography do not differ from those identified in the nonoperative setting. Patients with a clear indication for angiography on clinical grounds, apart from perioperative risk stratification, should be managed according to standard guidelines.
- The use of coronary computerized tomography angiography and calcium scoring to determine the presence and extent of CAD is a less invasive and lower risk alternative to cardiac catheterization. However, its utility in preoperative risk assessment has not been fully established.49
TREATMENT
Medications
β-Blockers
- Multiple, earlier, small trials suggested that perioperative β-blocker therapy improves cardiovascular outcome and reduces mortality.50–53
- More recent data, however, have questioned the purported benefits of perioperative β-blockade.54–57
- In particular, the large POISE trial, which confirmed a decrease in cardiac events (e.g., ischemia, atrial fibrillation, need for coronary interventions) with aggressive perioperative β-blockade, but also demonstrated a higher overall mortality, related to an increased rate of stroke and death from noncardiac complications.56
- An important difference between the studies showing effectiveness of perioperative β-blocker therapy and the POISE trial was the β-blocker regimen used. The POISE trial employed a relatively high dose of extended-release metoprolol beginning on the day of surgery. Prior studies, however, started a long-acting β-blocker days to weeks before surgery and titrated the dose preoperatively.
- In particular, the large POISE trial, which confirmed a decrease in cardiac events (e.g., ischemia, atrial fibrillation, need for coronary interventions) with aggressive perioperative β-blockade, but also demonstrated a higher overall mortality, related to an increased rate of stroke and death from noncardiac complications.56
- The most recent ACC/AHA guidelines have incorporated the newer results into the recommendations regarding perioperative β-blocker use.1,58
- β-blockers should be continued in patients receiving them chronically for clear indications. Abrupt withdrawal of long-term β-blockade is harmful.59
- In patients with three or more RCRI risk factors (e.g., DM, HF, CAD, renal insufficiency, CVA) or intermediate- to high-risk myocardial ischemia noted on preoperative testing, it may be reasonable to begin perioperative β-blockade.
- It is preferred that β-blocker therapy is initiated more than 1 day before surgery in order to assess clinical effectiveness, safety, and tolerability. The need for dose titration is controversial.60 β-blockers should not be started on the day of surgery.
- β-blockers should be continued in patients receiving them chronically for clear indications. Abrupt withdrawal of long-term β-blockade is harmful.59
α2-Agonists
- Previous data suggested that prophylactic use of α2-adrenergic agonists such as clonidine and mivazerol could reduce the risk of perioperative myocardial ischemia and death.61–63
- POISE-2, a recent large randomized clinical trial (RCT), failed to show any benefit of clonidine in decreasing perioperative morbidity or mortality. Clonidine did, however, increase the rate of nonfatal cardiac arrest and clinically significant hypotension.64
- The current ACC/AHA guidelines recommend against α2-agonists for the prevention of cardiac events.1
Angiotensin-Converting Enzyme Inhibitors
- At present, there are insufficient data regarding specific surgery types or patient subgroups that are most likely to benefit from holding ACE inhibitors perioperatively.
- A meta-analysis of available trials demonstrated more frequent transient intraoperative hypotension in patients taking ACE inhibitors or angiotensin-receptor blockers (ARBs), but no difference in perioperative cardiovascular events.65 A large retrospective study failed to find an association between ACE inhibitor use and hemodynamic findings.66 A very recent study supports the concern for hypotension and also suggests the possibility for an association with acute kidney injury.67 On the other hand, there is some evidence for poor outcomes in patients whose ACE inhibitors are discontinued abruptly and not resumed.68,69
- Continuation of ACE inhibitors or ARBs in the perioperative period is reasonable at present but new data are likely to refine this recommendation.1 The optimal timing of the last dose before surgery and the first postoperative is uncertain at this time.
Statins
- Observational data support a reduced rate of MACE in patient undergoing noncardiac surgery.70–73
- The effect may be more notable in those undergoing vascular surgery.74–76 It is reasonable to initiate statin therapy for anyone prior to vascular surgery.1
- Perioperative initiation of statins may also be considered in patients with clinical indications who are scheduled for elevated risk procedures.1
- Statin therapy should be continued in those currently taking statins.1
Revascularization
- The utility of preoperative revascularization in reducing perioperative cardiac complications continues to be uncertain. The combined morbidity and mortality of coronary revascularization and the planned noncardiac surgery must be considered in the context of a patient’s overall health, functional status, and long-term prognosis before proceeding with either.1
- Revascularization before vascular surgery has not demonstrated improved outcomes in RCTs.77–79 This includes the controversial DECREASE-V study.78,79 Left main disease may be an exception.80
- According to the most recent ACC/AHA guidelines, revascularization before noncardiac surgery should be limited to patients for whom revascularization is clinically indicated based on existing practice guidelines.1
- If preoperative PCI is necessary, the urgency of the noncardiac surgery, as well as the risk of bleeding and ischemic events, including stent thrombosis, associated with the surgery in a patient taking dual antiplatelet therapy (DAPT) is important to consider in determining optimal timing, stent, and antiplatelet strategy.
- The ACC/AHA recommends DAPT with aspirin (ASA) 81 mg daily and a P2Y12 receptor blocker (clopidogrel 75 mg daily, prasugrel 5 to 10 mg daily, or ticagrelor 90 mg bid) for a minimum duration of:1,81
- At least 1 month in patients receiving bare metal stents (BMS).
- 12 months in patients receiving drug-eluting stents (DES).
- At least 1 month in patients receiving bare metal stents (BMS).
- BMS and DES have similar rates of early stent thrombosis, and the risk is greatest in the first 4 to 6 weeks following implantation.82,83 This is largely attributed to the cessation of DAPT in the perioperative period. The risk of in-stent restenosis, however, is higher with BMS than DES.84,85
- Elective procedures with significant risk of bleeding should be postponed until patients have completed the minimum duration of DAPT. Some data suggest that in newer-generation DES, the risk of stent thrombosis is stabilized by 6 months after implantation.1 However, 12 months is still preferred due to the risk, albeit low, of very late stent thrombosis.
- If noncardiac surgery is time sensitive or the risk of bleeding is high, consideration should be given to balloon angioplasty or BMS placement. If coronary revascularization is imperative prior to urgent or emergent surgery, CABG may also be considered.1 For angioplasty alone, 2 to 4 weeks delay is recommended, though the event rates appear to be considerably lower.86,87
- For patients who have received coronary stents and must undergo procedures that mandate the discontinuation of P2Y12 receptor blocker therapy, it is recommended to continue ASA if possible and to restart DAPT as soon as feasible after surgery.1
- DAPT should not be discontinued without first consulting the patient’s cardiologist. See Figure 2-2 for an algorithm for antiplatelet management in patients with PCI and noncardiac surgery.1
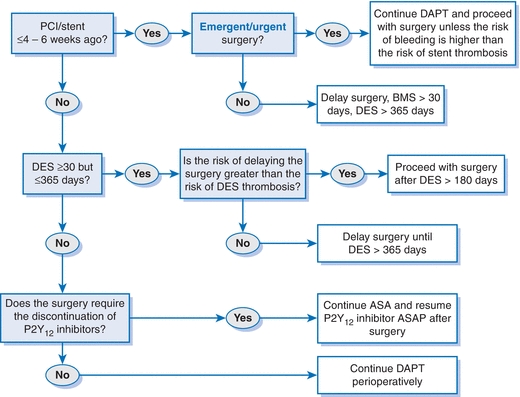
Figure 2-2 Perioperative antiplatelet therapy for patients with PCI. Modified from Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 2014;64:e77–e137.
SPECIAL CONSIDERATIONS
Hypertension
- DBP <110 mm Hg is does not appear to be an independent predictor of perioperative MACE, though evidence is limited.88 A specific statement cannot be made about SBP due to a lack of data.
- Higher preoperative BPs are thought to be associated with exaggerated BP responses to anesthesia, reactions to noxious stimuli,, and overall BP lability. Weak data also suggest an association with MI, arrhythmias, and other complications. Whether or not lowering BP preoperatively mitigates this risk is unclear at this time.
- Patients receiving antihypertensive agents for longitudinal indications are generally advised to remain on their current medication regimen in the perioperative period.1
- Patients chronically on β-blockers should continue therapy to avoid tachycardia and rebound hypertension.
- Maintaining continuity of ACE inhibitors or ARBs in the setting of treatment for hypertension or HF is also recommended. If held on the day of surgery, these medications should be resumed as soon as clinically feasible postoperatively.65–69
- Patients chronically on β-blockers should continue therapy to avoid tachycardia and rebound hypertension.
Valvular Heart Disease
- Patients with preoperative suspicion for moderate to severe valvular abnormalities should be evaluated by echocardiography (if one has not been done in the past 1 year).1
- Symptomatic stenotic lesions are potentially more problematic than regurgitant lesions. Time permitting (i.e., the planned surgery is elective) therapy should follow established guidelines, including valvular repair or replacement as appropriate.1,89 Transcatheter aortic valve replacement may be an option for some patients felt to be poor candidates for standard surgical aortic valve replacement.1
- The perioperative risks associated with severe aortic and mitral stenosis are currently felt to be less than indicated by prior studies, which demonstrated a very high incidence of MACE.90–93 Therefore, these valvular abnormalities are no longer considered an absolute contraindication to urgent/emergent surgery.
- In patients with asymptomatic severe aortic or mitral stenosis, it may be acceptable to proceed with surgery and with appropriate intraoperative and postoperative hemodynamic monitoring.1
- Endocarditis prophylaxis is warranted only in specific clinical contexts (see Endocarditis Prophylaxis section).
Pacemakers and Implantable Cardioverter Defibrillators
- A major concern in the perioperative management of patients with cardiac implantable electronic devices (CIEDs) is the potential for interaction between the pacemaker or implantable cardioverter defibrillator (ICD) and monopolar electrocautery.
- Various errors may occur, ranging from resetting of the device to inadvertent reprogramming to unintentional discharge. Optimally, the device should be interrogated preoperatively and again postoperatively to ensure proper function.
- The most important precaution to take is direct communication between the physician managing the implanted device and the surgical team. In particular, they should discuss any electromagnetic interference that may occur during the planned procedure.1,94
- Continuous cardiac monitoring during the procedure should be done when an ICD has had its tachytherapy functions deactivated. A transcutaneous defibrillator must be available and the ICD must be reprogrammed after the surgery.1,94
Congestive Heart Failure
- Acute decompensated HF is a contraindication to surgery. Medical treatment for HF should be carefully optimized prior to elective noncardiac surgery (see Chapter 8).
- In a cohort study of 38,047 consecutive patients, perioperative morbidity and mortality was shown to be significantly higher in those with active HF compared to those with atrial fibrillation or CAD.95 Lower ejection fraction appears to be associated with increased risk.
Preoperative Pulmonary Evaluation
GENERAL PRINCIPLES
- Postoperative pulmonary complications contribute significantly to overall perioperative morbidity and mortality and are as common as cardiac complications.40,96
- A cost analysis performed by the NSQIP found pulmonary complications to be the most expensive of major perioperative medical complications, including cardiac, infectious, and thromboembolic, and also resulted in the longest length of stay.97
- The most significant complications include atelectasis, pulmonary infection, exacerbation of underlying lung disease, prolonged mechanical ventilation, and respiratory failure.
- The American College of Physicians practice guideline recognizes the following patient-related factors that increase the risk of postoperative pulmonary complications.40,96
- Age > 60
- History of chronic obstructive pulmonary disease (COPD)
- Congestive HF
- ASA class >II (Table 2-2)98
- Functional dependence
- Age > 60
- Other data support the following as additional patient-related risk factors: smoking, HF, malnutrition, obstructive sleep apnea (OSA), and pulmonary hypertension.99–102
- Factors that have not been consistently shown to be associated with postoperative pulmonary complications include controlled asthma and obesity.40
- Procedure-related risk factors include the following40,96,99–101:
- Surgical site: aortic, vascular, neurosurgery, head and neck, thoracic, and abdominal. Patients undergoing aortic aneurysm repair are at the highest risk for perioperative pulmonary complications. Low-risk procedures include hip surgery and gynecologic or urologic procedures.
- Emergency surgery.
- Prolonged surgery (>2.5 to 4 hours).
- General anesthesia.
- Use of long-acting neuromuscular blockers.
- Surgical site: aortic, vascular, neurosurgery, head and neck, thoracic, and abdominal. Patients undergoing aortic aneurysm repair are at the highest risk for perioperative pulmonary complications. Low-risk procedures include hip surgery and gynecologic or urologic procedures.
DIAGNOSIS
Clinical Presentation
- The focus should be on identifying the presence of patient-related risk factors.
- A thorough respiratory and smoking history should be obtained.
- Any current upper respiratory tract infection symptoms should be ascertained but they are not an absolute contraindication to surgery.
- Patients should be questioned about their general functional status and exercise tolerance.
- Attention should be paid to evidence of chronic lung disease such as increased anteroposterior dimensions of the chest, hyperresonance, diminished breath sounds, and the presence of adventitious lung sounds such as crackles, rhonchi, or wheezing.
- Signs of HF should also be sought, including rales, extra heart sounds, jugular venous distention, and peripheral edema.
- Body habitus may suggest the possibility of OSA and obesity hypoventilation syndrome. How aggressively to screen for OSA is an open question at present.
Diagnostic Criteria
- In contrast to the relatively well-defined risk assessment strategy for cardiovascular complications described above, there is no single, evidence-based algorithmic approach for perioperative pulmonary risk stratification for noncardiac surgery.
- The postoperative pneumonia risk index is presented in Table 2-4.99
- The respiratory failure risk index of Arozullah et al.103 is a validated model that can assist in predicting the rate of postoperative respiratory failure (Table 2-5). An analysis of a larger more representative sample of surgical patients resulted in the respiratory risk index that is predictive of ≥48 hours of ventilator dependence or unplanned reintubation. It consists of 28 independently associated variables and, therefore, is not likely to be used frequently in routine clinical practice.100
- The Canet Risk Index was more recently developed to help determine the incidence of perioperative pulmonary complications of any severity. This index, based on seven, independent risk factors, was derived from a relatively small cohort study, however, and has the disadvantage of including outcomes of minor clinical significance (e.g., new wheezing treated with bronchodilators).104
- Regardless of how risk is determined, those at increased risk are reasonable targets for strategies intended to mitigate the risk of postoperative pulmonary complications.
TABLE 2-4 Postoperative Pneumonia Risk Index
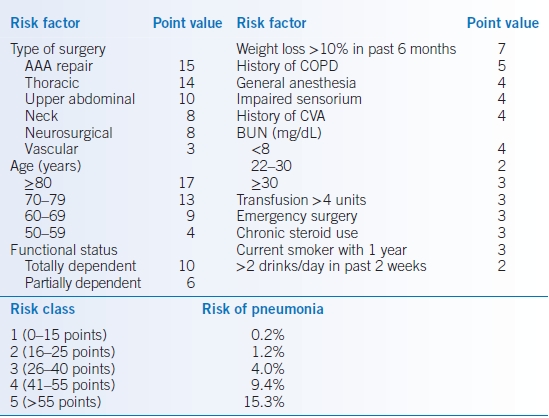
AAA, abdominal aortic aneurysm; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident.Modified from Arozullah AM, Khuri SF, Henderson WG, et al.; Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001;135:847–857.
TABLE 2-5 Respiratory Failure Risk Index
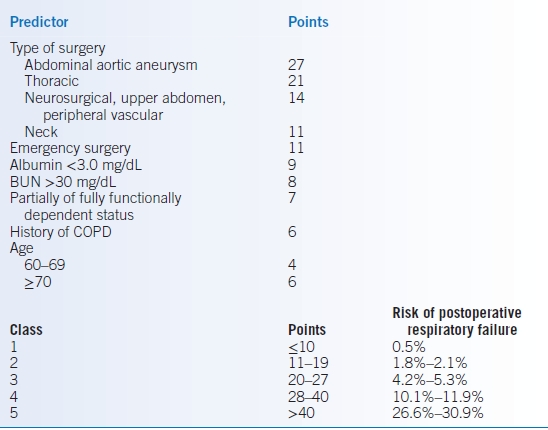
Modified from Arozullah AM, Daley J, Henderson WG, et al. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg 2000; 232:242–253.
Diagnostic Testing
Laboratory Studies
- A decreased serum albumin level is a potent predictor of pulmonary risk.40,96
- A level <3.5 mg/dL appears to be indicative of increased risk. It is recommended to measure albumin in patients clinically suspected to have hypoalbuminemia as well as those with one or more risk factors for perioperative pulmonary complications.
- At present, however, there is no conclusive evidence that enteral or parenteral nutritional supplementation decreases this risk.
- A level <3.5 mg/dL appears to be indicative of increased risk. It is recommended to measure albumin in patients clinically suspected to have hypoalbuminemia as well as those with one or more risk factors for perioperative pulmonary complications.
- Elevated blood urea nitrogen (BUN) and creatinine have been associated with pulmonary complications. Preoperative renal function testing is therefore reasonable.
- It is unclear whether arterial blood gas (ABG) results contribute to the estimate of perioperative pulmonary risk beyond the aforementioned clinically derived variables.
- There are no proven abnormal levels beyond which surgery is contraindicated.
- A preoperative ABG is only justified if otherwise clinically necessary.
- The primary purpose in most patients will be to serve as a baseline comparison for ABGs that may be obtained in the postoperative period.
- There are no proven abnormal levels beyond which surgery is contraindicated.
Imaging
- Many chest radiography (CXR) findings deemed abnormal are chronic and do not generally affect management.4,40,96,101,105,106 Furthermore, abnormal preoperative CXRs are often predicted based on the history and physical examination, and CXR only rarely provides unexpected information that influences perioperative management.
- Routine preoperative CXR in all patients is not recommended.
- Limited evidence suggests that preoperative CXR may be beneficial for patients with known cardiopulmonary disease, and those >50 years of age who are undergoing upper abdominal, thoracic, or abdominal aortic aneurysm surgery.96
Diagnostic Procedures
- The value of pulmonary function testing before lung resection and in determining candidacy for CABG is generally well accepted but no consensus exists on the utility of routine spirometry before extrathoracic surgery.40,96,101
- Preoperative spirometry may identify patients at higher risk of pulmonary complications. However, the data are mixed and spirometry has not been shown to be superior to history and physical examination in predicting perioperative risk.
- No prohibitive spirometric threshold has been established below which the risk of surgery is unacceptable.
- Pulmonary function testing is warranted for patients with a suspected but yet undiagnosed pulmonary condition for which evaluation would have also been indicated outside the context of surgery.
TREATMENT
Smoking Cessation
- Smoking is associated with an increased occurrence of postoperative pulmonary complications.40,101,102
- A clear benefit has been shown if patients abstain from smoking at least 4 weeks prior to scheduled surgery.101,107–109
- Previous concerns about a paradoxical increase in complications appear unwarranted, so all patients should be counseled to stop smoking even if <8 weeks from surgery.108
- Smoking cessation is discussed in detail in Chapter 45.
Obstructive Lung Disease Therapy
- Medical therapy should be optimized and elective surgery postponed, if possible, in the setting of acute exacerbations of COPD or asthma.
- The treatment of COPD and asthma is discussed in Chapter 16.
- See Perioperative Corticosteroid Management regarding COPD or asthma patients taking systemic steroids.
Postoperative Interventions
- Lung expansion maneuvers (i.e., incentive spirometry, deep breathing exercises, and continuous positive airway pressure [CPAP]) may reduce the risk of postoperative pulmonary complications.101,107 However, two recent meta-analyses have questioned the effectiveness of incentive spirometry and CPAP.110,111
- Patients with diagnosed OSA on CPAP therapy should continue it up to the day of surgery and during the hospitalization.
- A strategy of selective nasogastric tube placement after abdominal surgery rather than routine use has also been shown to decrease the risk of pulmonary complications.107,112
Perioperative Consideration in Liver Disease
GENERAL PRINCIPLES
- Patients with end-stage liver disease undergoing surgery are at high risk for perioperative morbidity and mortality.
- Likely related to decreased hepatic perfusion during anesthesia, patients with advanced liver disease have substantial risk of acute hepatic decompensation postoperatively.113
- The systemic effects of liver dysfunction can subsequently result in an increased frequency of other major complications as well, including severe coagulopathy, encephalopathy, acute respiratory distress syndrome, acute renal failure, and sepsis.
DIAGNOSIS
- Since significant liver disease is usually suspected clinically and the prevalence of unexpected liver enzyme abnormalities is low, routine laboratory screening for hepatic dysfunction is not recommended.114
- Patients with known or suspected liver disease should undergo a thorough evaluation of liver function including hepatic enzyme levels, albumin and bilirubin measurements, and tests of hemostasis.
- In patients with cirrhosis, higher Child-Pugh scores correlate with greater degrees of hepatic dysfunction and increasing perioperative morbidity and mortality.115
- Studies indicate that the Model of End-Stage Liver Disease (MELD) score may also be a reliable predictor of postoperative complications and mortality. The MELD score is discussed in detail in Chapter 30. Data suggest that a MELD score >14 more accurately predicts poor outcomes than does a Child class C.116
TREATMENT
- Elective surgery is contraindicated in patients with acute viral or alcoholic hepatitis, and should be delayed until recovery is documented. Patients with chronic hepatitis but without evidence of hepatic decompensation generally tolerate surgery well.
- The AGA Institute recommends the following management in cirrhotic patients116:
- For those with a MELD score <11, the postoperative mortality is adequately low to justify the risks of surgery.
- Patients with a MELD score of 12 to 19 should complete liver transplant evaluation prior to elective surgery so that they may proceed to urgent liver transplantation if necessary.
- Elective procedures should be postponed until after liver transplantation in patients with a MELD score ≥20.
- Others have suggested similar ranges, MELD <10, 10 to 15, and >15.117
- For those with a MELD score <11, the postoperative mortality is adequately low to justify the risks of surgery.
- Based on the high perioperative mortality rates in patients with advanced cirrhosis, nonsurgical alternatives should be strongly considered.
- For patients requiring surgery, steps should be taken to optimize the preoperative status including coagulopathy, thrombocytopenia, renal and electrolyte abnormalities, volume status, ascites, and encephalopathy. These subjects are discussed in detail in Chapter 30.
Perioperative Consideration in Kidney Disease
- Chronic kidney disease (CKD) is an independent risk factor for perioperative MACE, so patients with renal disease need appropriate cardiac risk stratification.1,11,18,39
- End-stage renal disease (ESRD) patients undergoing surgery possess a substantial risk of morbidity and mortality.118
- Most general anesthetic agents have no appreciable nephrotoxicity or effect on renal function other than that mediated through hemodynamic changes.119
- Every effort should be made to achieve euvolemia preoperatively in order to reduce the incidence of volume-related complications in the perioperative period.120
- Although this usually entails removing fluid, some patients may be hypovolemic and require hydration.
- Patients with CKD may need diuretic therapy before surgery.
- ESRD patients on dialysis should receive dialysis preoperatively. This is typically performed on the day prior to surgery but can be done on the day of surgery as well. The potential for transient electrolyte abnormalities, fluid shifts, and hemodynamic changes postdialysis must be considered, however.
- Although this usually entails removing fluid, some patients may be hypovolemic and require hydration.
- Hyperkalemia in the preoperative setting should be treated according to standard practice, particularly since the tissue breakdown occurring with surgery is likely to elevate the potassium level further (see Chapter 24).
- Platelet dysfunction is associated with uremia.
- The ability of preoperative bleeding time to predict postoperative hemorrhagic complications is questionable.121 Assessment of bleeding time prior to surgery is not routinely recommended in patients with CKD or ESRD.
- Patients with evidence of perioperative bleeding, however, should receive further evaluation and treatment (see Chapter 12).
- The ability of preoperative bleeding time to predict postoperative hemorrhagic complications is questionable.121 Assessment of bleeding time prior to surgery is not routinely recommended in patients with CKD or ESRD.
Perioperative Consideration in Diabetes Mellitus
GENERAL PRINCIPLES
- Hospitalized patients with diabetes and hyperglycemia are at increased risk for poor outcomes.122
- Data from critically ill/intensive care unit (ICU) patients initially suggested improved outcome with tight glycemic control. More recent trials using very stringent targets (e.g., 80 to 110 mg/dL) have failed to demonstrate a mortality benefit, possibly due to difficulty achieving these goals without raising the risk of severe hypoglycemia.123–126
- Although it is uncertain if improving glucose control in the non-ICU setting decreases morbidity or mortality, the robust association between hyperglycemia and surgical complications such as poor wound healing still makes glycemic control a priority in the perioperative setting.122
- Severe hypoglycemia (<40 mg/dL) is independently associated with in-hospital mortality and should be scrupulously avoided.127,128
TREATMENT
- Elective surgery in patients with uncontrolled DM should preferably be delayed until acceptable glycemic control has been achieved.
- Whenever possible, procedures should be scheduled for early morning to minimize prolonged fasting.
- Frequent monitoring of blood glucose levels is essential in all situations.
- Hypoglycemia must be avoided with the same vigor as hyperglycemia.
Target Glucose Levels
- The current American Diabetes Association inpatient guidelines take into account the most recent trial data regarding tight glycemic control and the risk of hypoglycemia.122
- For critically ill patients, therapy should be initiated for persistent hyperglycemia (threshold no >180 mg/dL) with a target glucose range of 140 to 180 mg/dL. Targets <110 mg/dL are not recommended. Insulin infusion protocols are preferred in such situations.
- In noncritically ill hospitalized patients, a preprandial goal of <140 mg/dL and random blood glucose levels <180 mg/dL is recommended.
- The optimal glucose range in postsurgical patients is uncertain but likely to be in a very similar range (e.g., 150 to 200 mg/dL). More stringent targets are ineffective (i.e., do not lower the rate of infection, cardiovascular events, renal failure, or death) and associated with increased episodes of hypoglycemia.129
- The publically reported Surgical Care Improvement Project (SCIP) quality metric for patients undergoing cardiac surgery is glucose <200 mg/dL at 6 am, the morning following surgery.
Type 1 Diabetes
- Some form of basal insulin is required at all times.
- On the evening prior to surgery, the regularly scheduled basal insulin dose should be continued. If taken in the morning, it is still recommended to give the basal insulin without dose adjustment.
- IV glucose (i.e., 5% dextrose-containing fluids) may need to be administered in the perioperative period to avoid hypoglycemia while the patient is NPO and until tolerance of oral intake is observed.
- For complicated surgeries or procedures requiring prolonged NPO status, a continuous insulin infusion will likely be necessary.
- Caution should be exercised with the use of subcutaneous insulin in the intraoperative and critical care settings, as alterations in tissue perfusion may result in variable absorption.
Type 2 Diabetes
- Diet controlled
- These patients can generally be managed without insulin therapy.
- Glucose values should be checked regularly. Elevated levels (>180 mg/dL) can be treated with intermittent doses of short-acting insulin.
- These patients can generally be managed without insulin therapy.
- Managed with oral therapy
- Short-acting sulfonylureas, as well as most other oral diabetic agents, should be held on the day of surgery.
- Metformin and long-acting sulfonylureas (e.g., glimepiride) should be withheld 1 day before scheduled surgical procedures.
- Metformin is generally held for at least 48 hours postoperatively. Renal function should be normal or at baseline prior to resuming metformin.
- Other oral diabetic agents can be resumed once patients are tolerating their preprocedure diet. This recommendation assumes such patients will be discharged from the hospital shortly; otherwise, oral hypoglycemic medications are generally inappropriate for most inpatients.
- As in the diet-controlled type 2 diabetics, blood sugar should be checked regularly and short-acting insulin therapy initiated if persistent hyperglycemia (>180 mg/dL) is noted.
- Most of these patients can be managed without an insulin infusion.
- Short-acting sulfonylureas, as well as most other oral diabetic agents, should be held on the day of surgery.
- Managed with insulin
- So-called sliding scale insulin is often ineffective as monotherapy.
- If it is anticipated that the patient will be able to eat postoperatively, basal insulin should be given on the morning of surgery.
- If given as long-acting insulin (e.g., glargine insulin) and the patient usually takes the dose in the morning, 80% to 90% of the routine dose can be given.
- If the patient uses intermediate-acting insulin (e.g., NPH), half to two-thirds of the usual morning dose is given to avoid periprocedural hyperglycemia.
- If given as long-acting insulin (e.g., glargine insulin) and the patient usually takes the dose in the morning, 80% to 90% of the routine dose can be given.
- Patients undergoing major procedures may require an insulin infusion perioperatively. In these cases, glucose-containing IV fluids with supplemental potassium should be administered concomitantly to avoid hypoglycemia and hypokalemia, respectively.
- The baseline insulin regimen can typically be resumed once oral intake is reestablished.
- So-called sliding scale insulin is often ineffective as monotherapy.
Perioperative Antiplatelet and Anticoagulation Management
ANTIPLATELET AGENTS
- Patients taking ASA for secondary prevention may continue it if having a minor dental, dermatologic, or cataract procedures.130
- Early use of ASA improves outcomes after PCI and CABG.131 For patients undergoing PCI or CABG, ASA should be continued without interruption.130
- For patients undergoing CABG who are taking DAPT, the P2Y12 receptor blocker should be held 5 to 7 days before surgery.130
- If possible, ASA and a P2Y12 receptor blocker (clopidogrel, prasugrel, or ticagrelor) should be continued perioperatively in patients with recent coronary stents and who are undergoing noncardiac surgery. If surgery can be safely postponed until the minimal period of DAPT is complete (4 to 6 weeks BMS, 12 months DES), it should be.1,81,130 Refer to the Revascularization section above for a detailed discussion. If the minimum time as has elapsed and the patient continues DAPT, then the P2Y12 receptor blocker can be stopped starting 5 to 7 days preoperatively.
- The most recent American College of Chest Physicians (ACCP) guidelines recommend continuing ASA in patients who are at moderate to high risk for cardiovascular events.130 But this recommendation came before the results of the POISE-2 trial. ASA is not indicated for patients at low risk.
- The recent POISE-2 trial enrolled over 10,000 patients at risk for cardiovascular complications who were going to undergo noncardiac surgery.
- Inclusion criteria selected for those with CVD (i.e., history of CAD, PAD, CVA, or the need for major vascular surgery) or with several factors known to increase risk. Those within 1 year or 6 weeks of receiving a DES or BMS, respectively, were excluded. Very few patients who had a prior stent and few with a history of CAD were included.
- Nearly half of the patients were already on a daily ASA regimen, which they stopped taking at least 3 days before surgery per the study protocol. In those patients who were randomized to the placebo group, there was no associated increase in thrombotic events related to sudden withdrawal of ASA.
- Patients were randomized to receive either ASA or placebo just before surgery and for 30 days after.
- There was no difference in the primary outcome of death or nonfatal MI between the ASA and placebo groups. There was, however, a higher rate of major bleeding in the ASA group.132
- These results suggest that patients at risk for CVD who are taking ASA for prevention of cardiovascular events should discontinue it at least 3 days prior to surgery.132 Because the number of patients who had previously undergone PCI is small, it is difficult to draw firm conclusions about this group.133
- Inclusion criteria selected for those with CVD (i.e., history of CAD, PAD, CVA, or the need for major vascular surgery) or with several factors known to increase risk. Those within 1 year or 6 weeks of receiving a DES or BMS, respectively, were excluded. Very few patients who had a prior stent and few with a history of CAD were included.
- The ACC/AHA guideline, which does include the results of the POISE-2 trial, states that it still may be reasonable to continue ASA in patients at high risk for CAD or CVA when the risk of cardiovascular events outweighs the potential risk of increased bleeding, particularly those with a prior stent (Fig. 2-2).1
ANTICOAGULANTS
- In determining an appropriate anticoagulation strategy, the benefit of continued treatment must be weighed against the risk of bleeding for each individual patient and procedure.
- Proceeding directly to surgery is generally acceptable if the INR ≤ 1.5.
- Some procedures may be safely performed with an INR of 2.0 to 3.0 (e.g., endoscopy without biopsy, dental procedures, and skin biopsies). For dental procedures, the antifibrinolytic oral mouthwash tranexamic acid may be used.130
- If interruption of warfarin therapy is necessary, it should be discontinued 4 to 5 days before the procedure, allowing the INR to drift below 1.5.130,134 Warfarin should then be resumed 12 to 24 hours after surgery and when there is adequate hemostasis.130
- With regard to the novel oral anticoagulants (NOACs), including the direct thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban and apixaban, there are no data available to guide perioperative management.130 Given the lack of a specific reversal agent, however, discontinuation of NOACs ≥48 hours prior to surgery is recommended.1
- If a temporary interruption of anticoagulation is unacceptable (e.g., mechanical heart valve, atrial fibrillation, or high/moderate risk VTE), parenteral bridging anticoagulation (i.e., low molecular weight heparin [LMWH] or unfractionated heparin [UFH]) is warranted but should be discontinued 4 to 24 hours before the procedure, depending on the half-life of the drug.130
- LMWH is favored over UFH in patients with normal renal function since it is easier to administer and does not require routine testing of PT, aPTT, or anti-Xa levels.
- Following high-bleeding-risk surgery bridging anticoagulation can be resumed in approximately 48 to 72 hours. This would include urologic, cardiac, intracranial, and spinal procedures; surgery on highly vascular organs (e.g., kidney, spleen, liver); bowel resection; major surgery with extensive tissue injury (e.g., joint arthroplasty, cancer procedures, reconstructive plastic surgery); pacemaker and ICD placement; and resection of colonic polyps (usually sessile polyps >1 to 2 cm long).
- Following non–high-bleeding-risk surgery, anticoagulation can be resumed at 24 hours.
- LMWH is favored over UFH in patients with normal renal function since it is easier to administer and does not require routine testing of PT, aPTT, or anti-Xa levels.
Perioperative Corticosteroid Management
GENERAL PRINCIPLES
- Surgery is a potent activator of the hypothalamic-pituitary axis (HPA). Patients with adrenal insufficiency may lack the ability to respond appropriately to surgical stress.
- Additionally, patients on chronic corticosteroid therapy for indications other than adrenal dysfunction are at risk of developing perioperative adrenal insufficiency.
- The subtype of adrenal insufficiency has implications on management.
- Tertiary adrenal insufficiency due to exogenous corticosteroid administration is the most common adrenal problem encountered. These patients should have intact mineralocorticoid function and therefore require only glucocorticoid supplementation.
- Likewise, secondary adrenal insufficiency should not result in mineralocorticoid deficiency. The possibility of deficits in other hormones due to pituitary disease should be considered.
- Primary adrenal insufficiency requires replacement of both mineralocorticoids and glucocorticoids.
- Tertiary adrenal insufficiency due to exogenous corticosteroid administration is the most common adrenal problem encountered. These patients should have intact mineralocorticoid function and therefore require only glucocorticoid supplementation.
- The dose and duration of exogenous corticosteroids required to produce clinically significant tertiary adrenal insufficiency is highly variable, but general principles can be outlined135:
- Daily therapy with ≤5 mg of prednisone (or equivalent), alternate day corticosteroid therapy (<10 mg of prednisone), and any dose given for <3 weeks should not result in clinically significant adrenal suppression. Testing is not necessary in these patients and supplemental perioperative steroids are not warranted.
- Patients currently receiving >20 mg/day of prednisone (or equivalent) for >3 weeks, as well as those cushingoid in appearance, can be expected to have suppression of adrenal responsiveness, and, therefore, testing is not necessary.
- The function of the HPA cannot be readily predicted in patients currently taking prednisone doses of 5 to 20 mg for >3 weeks or who have received >5 mg/day for >3 weeks anytime within the prior year. These patients should be tested for adrenal responsiveness.
- While uncommon, HPA suppression can occur with high-dose inhaled corticosteroids. One can consider testing adrenal responsiveness in patients taking >800 μg of fluticasone/day or >1,500 μg/day of another inhaled corticosteroid.136,137
- Daily therapy with ≤5 mg of prednisone (or equivalent), alternate day corticosteroid therapy (<10 mg of prednisone), and any dose given for <3 weeks should not result in clinically significant adrenal suppression. Testing is not necessary in these patients and supplemental perioperative steroids are not warranted.
DIAGNOSIS
- For patients in whom clinical prediction of adrenal function is difficult, a morning (AM) cortisol or a cosyntropin stimulation test can be performed.
- An AM cortisol level of >10 μg/dL is indicative of a very low likelihood of HPA suppression, while an AM cortisol level of <5 μg/dL (>24 hours after the last dose of exogenous steroid) is suggestive of significant suppression. Values between 5 and 10 μg/dL are nondiagnostic and require further investigation with a cosyntropin stimulation test.138
- The short cosyntropin stimulation test is as follows: 250 μg IV or IM cosyntropin is given and plasma cortisol is measured 30 minutes later. The normal response is a stimulated plasma cortisol >20 μg/dL.
TREATMENT
- It is generally agreed that patients with known or expected adrenal insufficiency undergoing more than minor surgery should be treated with perioperative glucocorticoids.
- If HPA axis status is uncertain and there is inadequate time to perform a cosyntropin stimulation test, preoperative corticosteroids should be administered empirically.
- The following guidelines are based on extrapolation from small studies in the literature, expert opinion, and clinical experience135:
- Minor surgical stress (e.g., colonoscopy, cataract surgery, or inguinal hernia repair): Give 25 mg hydrocortisone or 5 mg methylprednisolone IV on the day of the procedure only. Some suggest that no so-called stress doses steroids are needed in these circumstances and the patient should simply take their usual morning dose of steroids.
- Moderate surgical stress (e.g., cholecystectomy, hemicolectomy, or joint replacement): Give 50 to 75 mg hydrocortisone or 10 to 15 mg methylprednisolone IV on the day of surgery and taper quickly over 1 to 2 days to the usual dose.
- Major surgical stress (e.g., cardiothoracic surgery, aortic aneurysm repair, or Whipple procedure): Give 100 to 150 mg hydrocortisone or 20 to 30 mg methylprednisolone IV on the day of the procedure and taper to the usual dose over the next 1 to 2 days.
- Critically ill patients requiring emergent surgery (e.g., septic or cardiogenic shock): Give 50 to 100 mg hydrocortisone IV every 6 to 8 hours or 0.18 mg/kg/hour as a continuous infusion plus 0.05 mg/day of fludrocortisone until shock resolves. Then gradually taper the dose, monitoring vital signs and serum electrolytes closely.
- Minor surgical stress (e.g., colonoscopy, cataract surgery, or inguinal hernia repair): Give 25 mg hydrocortisone or 5 mg methylprednisolone IV on the day of the procedure only. Some suggest that no so-called stress doses steroids are needed in these circumstances and the patient should simply take their usual morning dose of steroids.
- Additional mineralocorticoid supplementation for patients with primary adrenal insufficiency may or may not be necessary, depending on the dose and mineralocorticoid potency of the corticosteroid given.
- A recent systematic review, including nine small studies, suggested that stress dose steroids may not be routinely required in the perioperative period for patients on therapeutic doses, so long as they continue to receive their usually daily dose.139
Other Medication Adjustments in Perioperative Period
THYROID HORMONE REPLACEMENT
- Patients with hypothyroidism who appear clinically euthyroid and are receiving thyroxine treatment can safely skip this medication for several days owing to its long half-life.
- Patients should restart therapy once they can take PO.
- In patients who are unable to resume oral intake within 5 to 7 days, IV thyroxine should be given at approximately 80% of the oral dose.140
ANTICONVULSANTS
- Elective surgery is generally avoided in patients with poorly controlled seizures.
- If surgery is necessary, anticonvulsants should be given parenterally until the patient is able to resume taking medications by mouth.
- When IV or IM preparations are unavailable, substitutes such as phenytoin, levetiracetam, or phenobarbital may be used, depending on seizure type.
- Phenytoin has a relatively long half-life and therefore a single dose may be held safely.
PSYCHIATRIC MEDICATIONS
- Benzodiazepines should be continued postoperatively in patients chronically receiving them, given the risk for acute, life-threatening withdrawal with abrupt cessation.
- Selective serotonin reuptake inhibitors (SSRIs) can safely be given in the perioperative period. Observational studies suggest an association of SSRIs with a very small increased risk of bleeding, but whether this should impact perioperative medication management is unknown.141
- Tricyclic antidepressants (TCAs) have significant anticholinergic and α-adrenergic blocking properties, in addition to a high potential for drug-drug interactions. It is preferable to hold TCAs several days prior to elective surgery.
- Monoamine oxidase inhibitors (MAOIs) have the potential for severe drug interactions, and, therefore, must be stopped at least 2 weeks prior to elective surgery.
- Antipsychotics can typically be continued without adverse effects. However, the clinician should be mindful of the QT prolongation that can occur with these medications and should consider discontinuation if a preoperative ECG demonstrates a prolonged QT interval.
- Lithium has a narrow therapeutic window. Serious toxicity can occur when overdosed. Lithium can cause fluid and electrolyte abnormalities and prolong the effects of anesthetic and neuromuscular blocking agents. Lithium should be held 1 to 2 days preoperatively and restarted once the patient is reliably taking PO.
HERBAL MEDICATIONS
- It is important to ask patients specifically about the use of alternative or herbal preparations, as many will not report these when asked for a medication list.
- All of the following herbal remedies should be stopped before surgery142–144:
- Feverfew, ginger, and gingko can cause platelet dysfunction and increase the risk of perioperative bleeding.
- Valerian root potentiates the effects of sedatives and anxiolytics.
- St. John’s wort may have MAOI-like activity.
- Toxic effects of ma huang include hypertension, arrhythmias, and myocardial ischemia.
- Feverfew, ginger, and gingko can cause platelet dysfunction and increase the risk of perioperative bleeding.
Prophylactic Measures
VENOUS THROMBOEMBOLISM PROPHYLAXIS
- VTE prophylaxis is generally relevant only in the inpatient setting. However, because of its importance, the topic will be briefly covered here.
- VTE encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE). Prophylaxis is of paramount concern as DVT/PE remains the leading cause of preventable in-hospital mortality.
- The 2012 ACCP guidelines divide patients undergoing surgery into very low, low, moderate, or high risk groups.145,146 Although there have been many attempts to accurately quantitate these risks, no single method has been found to be universally acceptable. The Caprini VTE risk scoring model is presented in Table 2-6.145,147 The Patient Safety in Surgery Study score can also be used but pertains only to surgical patients and includes factors generally outside the purview of the internist (e.g., wound class and surgical relative value units).148 Regardless of the model used, the risk of VTE increases with the number of risk factors and point total.
- As seen in Table 2-6, patients at very low risk do not require specific prophylaxis. Those at low risk should receive mechanical prophylaxis. Those at high risk should receive pharmacologic and mechanical prophylaxis.145 A validation study of the Caprini model in a surgical population indicates that those with >8 points are at especially high risk of VTE, with mean risk >6%.149
- A number of pharmacologic agents are available for VTE prevention in surgical patients, including UFH, LMWH, fondaparinux, warfarin, dabigatran, rivaroxaban, and apixaban. The selection of an appropriate prophylactic agent depends on its efficacy and safety, the risk of bleeding, as well as patient comorbidities and preferences.
- VTE prophylaxis should commence either before or shortly after surgery, and at minimum be continued until the patient is ambulatory.
- Of note, extended VTE prophylaxis (usually between 10 and 35 days) is offered postoperatively to patients at highest risk for DVT/PE, particularly those who have undergone major orthopedic, oncologic, or abdominal surgery.147,149
TABLE 2-6 Caprini VTE Risk Scoring Model and the Risk of VTE
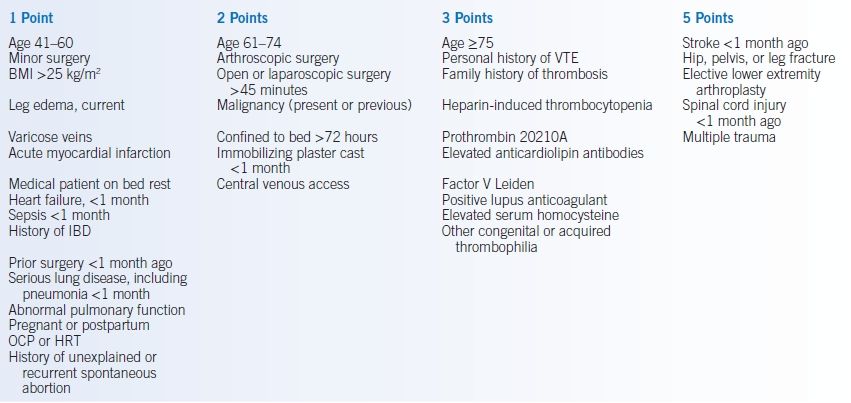
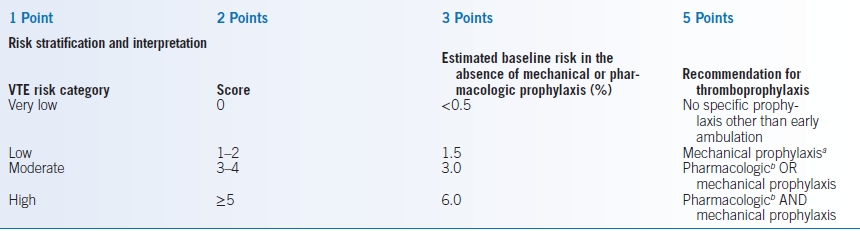
aIntermittent pneumatic compression favored over graduated compression stockings.
bPresuming the patient is not at high risk for major bleeding complications. Low molecular weight heparin generally preferred over unfractionated heparin.
Warfarin and other oral anticoagulants alternatives for total knee and hip replacement.BMI, body mass index; IBD, inflammatory bowel disease; OCP, oral contraceptive pills; HRT, hormone replacement therapy; VTE, venous thromboembolism.Data from Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e227S–e277S; Caprini JA. Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg 2010;199:S3–S10.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


