Cardiovascular System
Jochen K. Lennerz
John D. Pfeifer
HEART
I. NORMAL ANATOMY. The normal weight of the adult heart is 300 to 350 g (male) and 250 to 300 g (female). Cardiomegaly above a critical weight of 500 g is associated with ischemic changes (see later) and is termed cor bovinum. The normal ventricular thickness is 0.3 to 0.5 cm on the right and 1.2 to 1.5 cm on the left (e-Fig. 9.1),* measured at the base of the papillary muscles (Fig. 9.1). The heart is composed of three layers: the epicardium (including the serous or visceral pericardium, and the main branches of the coronary arteries), the muscular myocardium, and the endocardium (with an ill-defined subendocardial layer that contains many Purkinje fibers).
Microscopically, the normal myocardium is a functional syncytium of myocardial fibers (cardiac myocytes) that have centrally located nuclei (e-Fig. 9.1). Cardiac myocytes are a specialized form of striated muscle; faint dark eosinophilic intercalated discs between the myocytes form the mechanical and electrical couplings. Numerous capillaries with sparse interstitial tissue are found between the myocardial fibers (e-Fig. 9.1).
The atrioventricular valves (mitral and tricuspid) are composed of an annulus, leaflets, chordae tendineae, and papillary muscles. The semilunar valves (aortic and pulmonic) are composed of three cusps (each with a sinus), which meet at the three commissures (corpora arantii, e-Fig. 9.2). Valves are relatively avascular, and are lined by endothelial cells on a thin layer of collagen and elastic tissue on the atrial/arterial side, a thicker layer of dense collagen on the ventricular side, and loose myxoid connective tissue (zona spongiosa) in between. The fibrous and spongiotic regions are normally of equal thickness (e-Fig. 9.2).
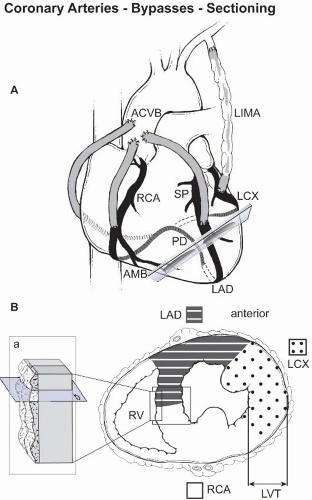
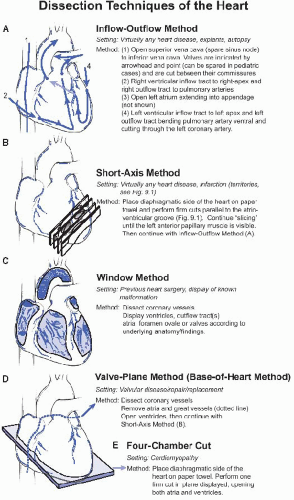
The conduction system is composed of specialized myocytes, with fewer intercalated discs and higher glycogen content. Masson trichrome, Verhoeff-van Gieson, and Alcian Blue stains can be used to demonstrate the conduction system (e-Fig. 9.3). Exact knowledge of the topographic anatomy and correct sampling techniques are paramount (e-Fig. 9.4).
II. GROSS EXAMINATION AND TISSUE HANDLING
A. Endomyocardial biopsies are usually taken via a right-sided cardiac catheter; the most common indications are monitoring of heart transplant rejection, and grading of Adriamycin toxicity. To avoid sampling errors, a minimum of three, preferably four, samples of myocardium are recommended (e-Fig. 9.5). The tissue fragments should be counted and measured during gross examination; their color and consistency should be noted. The tissue should be placed between foam pads or wrapped in filter paper for routine processing. Examination of at least three levels is recommended; some laboratories keep the intervening sections for additional stains if required to assess myocyte damage and fibrosis. Histologically, an adequate biopsy contains at least 50% myocardium, excluding previous biopsy sites (e-Fig. 9.5). Occasional cases require fresh frozen tissue or glutaraldehyde fixation for special techniques such as molecular diagnostics
or electron microscopy (see later), respectively. Adipose tissue between myocardiocytes is a normal finding and does not indicate ventricular perforation (e-Fig. 9.5).
B. Cardiac valves are often removed because of calcific degeneration or perforation as a sequela of bacterial endocarditis (e-Fig. 9.2). Most valves are received in fragments; if possible, the description should include the distribution of vegetations (e-Fig. 9.6) and presence or absence of non-surgery-related leaflet destruction. In cases of calcific degeneration, slow acid decalcification after fixation may be necessary. Sections are taken from the free edge to the annulus.
Prosthetic valves are typically removed because of thrombosis, anastomotic or valvular leakage, mechanical failure, or infection. Evaluation of the prosthetic valve ring attachment is, therefore, critical as infective endocarditis typically affects this region. For most mechanical heart valves, it is not possible to submit any tissue for histology, unless vegetations are present. For bioprosthetic valves, however, the valve cusp is submitted.
Valves from patients treated with the appetite-suppressant drug Fen-Phen (a combination of fenfluramine and phentermine) show patterns of changes that resemble carcinoid valve disease with superficial layers of myofibroblastic proliferation on otherwise normal valve architecture.
C. Myomectomy specimens, from ventricular aneurysm repair or septal myomectomy procedures should be measured, weighed, and sectioned at 3-mm intervals, perpendicular to the endocardial surface (Fig. 9.1). All layers of the heart should be described. For cardiac tumors, appropriate sections should assess the inked specimen resection margins (see later).
D. Heart explant specimens should be weighed, described, and dissected as outlined (Fig. 9.2). In addition, the valves (circumference or diameter) and walls (Fig. 9.1) should be measured. The septal and ventricular configuration (concentric vs. dilatative ventricular hypertrophy) should be described. Usually the inflow tract of the donor heart is dissected to match the recipient’s anatomy; thus, fragments of donor tissue are frequently submitted in the same container.
III. DIAGNOSTIC FEATURES OF COMMON DISEASES OF THE HEART
A. Disorders of the Endocardium
1. Infective endocarditis is characterized by bacterial colonization of the valve forming vegetations that are red, irregular (e-Fig. 9.6), and composed of granulation tissue and thrombus; their friability explains the propensity for associated septic embolization (e-Fig. 9.2). The myocardium is typically not involved. Staphylococcus aureus typically produces acute endocarditis, whereas Streptococcus viridans produces subacute endocarditis. Several organisms normally found in the oral cavity are also causative, and have been referred to as the gram-negative HACEK organisms (Hemophilus aphrophilus, Actinobacilus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingii). Staphylococcus epidermidis also causes infective endocarditis, more common in the setting of prosthetic valves. Healed infective endocarditis leaves residual valve damage, often fenestrations, usually with a hemodynamic jet lesion and adjacent endocardial fibrosis.
2. Nonbacterial thrombotic endocarditis (marantic endocarditis) produces small (rarely >0.5 cm), pink, bland, and sterile vegetations attached to the valve surface at the lines of closure (e-Fig. 9.6). It is typically seen in cachectic patients with a hypercoagulable state (e.g., Trousseau syndrome).
3. Libman-Sacks endocarditis is seen in 4% of cases of systemic lupus erythematosus and is characterized by flat, pale tan, spreading bands of vegetations located on both surfaces of the valves or chordae tendineae (e-Fig. 9.6). Affected, in order of frequency, are the tricuspid, mitral, pulmonic, and aortic valves.
4. Rheumatic heart disease (RHD) is a sequela of rheumatic fever (RF) caused by Streptococcus pyogenes (group A or β-hemolytic streptococcus). Aschoff nodules (ANs) are a characteristic feature and appear as interstitial collections of plump mononuclear cells with occasional neutrophils arranged in a granuloma-like formation, although the presence or the number of ANs does not correlate with clinical course or activity of the rheumatic process. The most characteristic cellular component of ANs is the Aschoff giant cell, which has two or more nuclei with prominent nucleoli; another characteristic feature is the presence of Anitschkow cells, which are mononuclear histiocytes that are often arranged in a palisade around the center of the granuloma. The macroscopic pattern is variable (e-Fig. 9.6).
The valvular disease characteristic of chronic RHD is usually the result of multiple recurrent episodes of acute RF, and typically develops many decades after the initial insult. RHD is the most common cause of mitral stenosis, and in up to 75% of cases, the mitral valve is the only valve affected. In 25% of cases, the mitral and aortic valves are affected. Progressive fibrosis leads to thickening of the valve and chordae that eventually leads to fusion of the mitral leaflets at the commissures, producing the classic “fish mouth” appearance.
5. Endocardial fibroelastosis is an uncommon condition that can result in restrictive cardiomyopathy. Pearly-white fibroelastic thickening, caused by accumulation of collagen and elastic fibers typically in the left ventricular endocardium (e-Fig. 9.3), is often associated with aortic valve obstruction. The disease occurs either focally or diffusely in children from birth to 2 years of age.
6. Loeffler endocarditis is also known as fibroelastic parietal endocarditis with blood eosinophilia. Classically, three stages (acute necrotic myocarditis, organizing thrombus, and endomyocardial fibrosis) are distinguished. The cardiac lesions are associated with dense eosinophilic infiltration of other organs, and the disease is usually rapidly fatal.
B. Disorders of the Valves
1. Myxoid change is stromal accumulation of glycosaminoglycans as a sign of degeneration (e-Fig. 9.2). The layered architecture is preserved; if the architecture is absent or distorted, the differential diagnosis should include RHD. The chordae tendineae are thinned and elongated in myxomatous degeneration, whereas in RHD they are shortened and thickened.
2. Mitral valves are removed for acquired post-inflammatory stenosis (e.g., RHD) and may show commissural fusion, cusp scarring, and dystrophic calcification (e-Fig. 9.2). RHD vegetations are composed mainly of fibrin and are usually no more than 2 mm in size. Cases of mitral insufficiency or myxomatous degeneration show a floppy valve with redundant and ballooned leaflets with abundant myxoid change.
3. Tricuspid valves are most commonly removed for insufficiency or infective endocarditis.
4. Aortic valves are removed for stenosis and are typically heavily calcified, sometimes with commissural fusion (senile calcific aortic stenosis), postinflammatory scarring, or calcification due to a congenitally bicuspid valve (present in 1% of the population).
5. Pulmonary valves are usually excised because of stenosis due to congenital heart disease (most commonly as a component of tetralogy of Fallot).
C. Disorders of the Myocardium
1. Myocarditis is the underlying etiology in about 10% of patients with newonset cardiac dysfunction. If not fatal, myocarditis often proceeds to dilated cardiomyopathy. Findings in subsequent biopsies, using the first specimen as a reference point, include ongoing/persistent myocarditis, resolving/healing
myocarditis (damage substantially reduced), and resolved/healed myocarditis (damage no longer present). The so-called Dallas criteria (Hum Pathol. 1987;18:619) are often used to categorize myocarditis mainly on histopathologic findings, although it has been suggested that the criteria are no longer adequate. The WHO defines myocarditis as a minimum of 14 infiltrating leukocytes per square millimeter, preferably T-cells, with as many as four macrophages (also known as the Marburg criteria; see Circulation. 1996;93:841). The diagnosis of active myocarditis classically requires the presence of an inflammatory infiltrate (usually lymphocytic) and myocyte necrosis/degeneration or damage not characteristic of an ischemic event; borderline myocarditis indicates the absence of necrosis or damage and can be applied to any form of inflammatory infiltrate.
a. Primary viral myocarditis accounts for most cases of myocarditis in developed countries. Cardiac involvement typically follows the primary viral infection by several days. The most commonly associated agents are enteroviruses (Coxsackie A and B), adenovirus, echovirus, and poliovirus, influenza viruses A and B, and HIV. The infiltrate is composed mainly of lymphocytes with associated myocyte damage (e-Fig. 9.7). Eosinophils are typically not seen. Primary viral myocarditis includes four clinical pathological manifestations: fulminate, chronic active, eosinophilic, and giant cell myocarditis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree