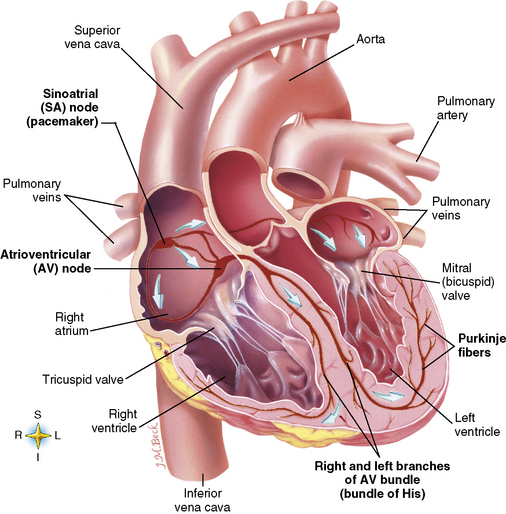
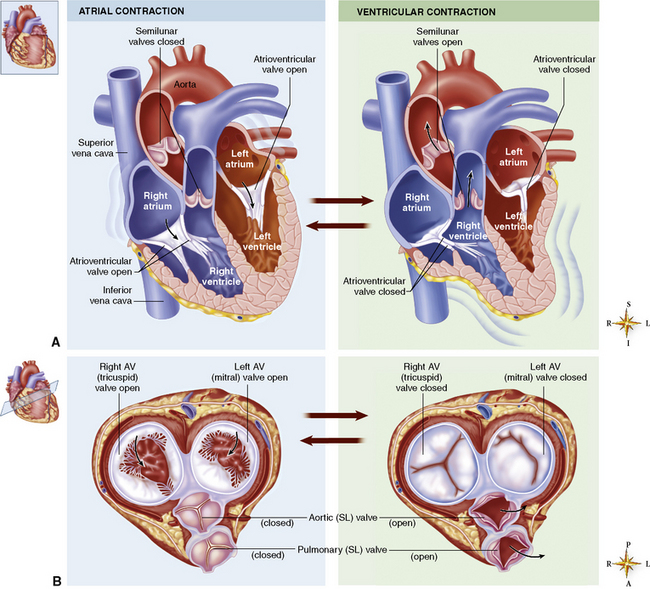
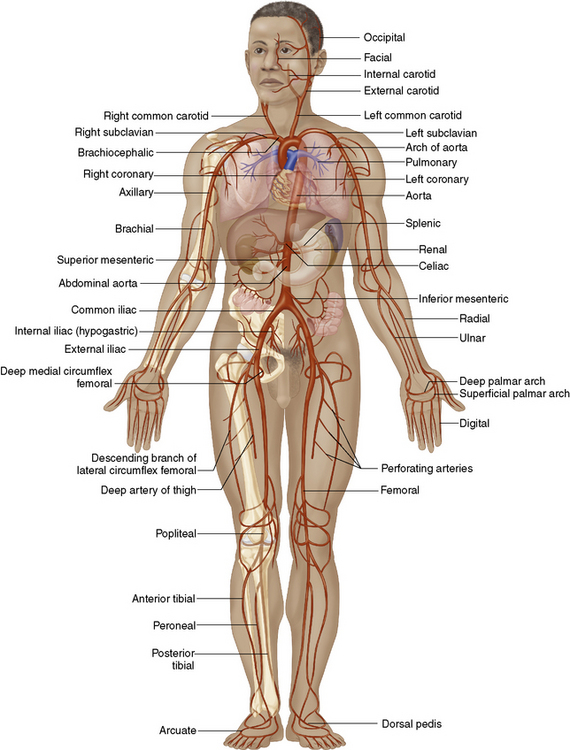
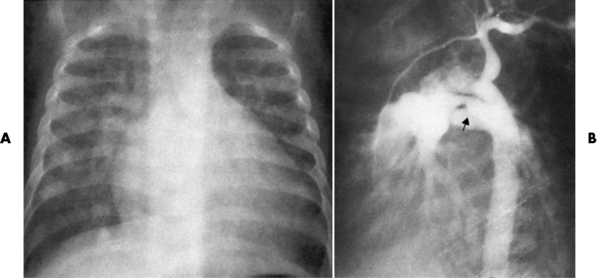
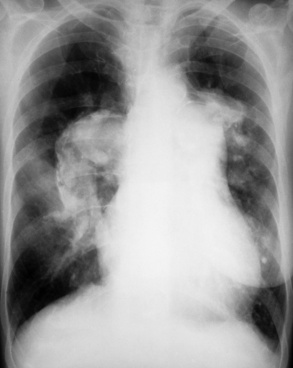
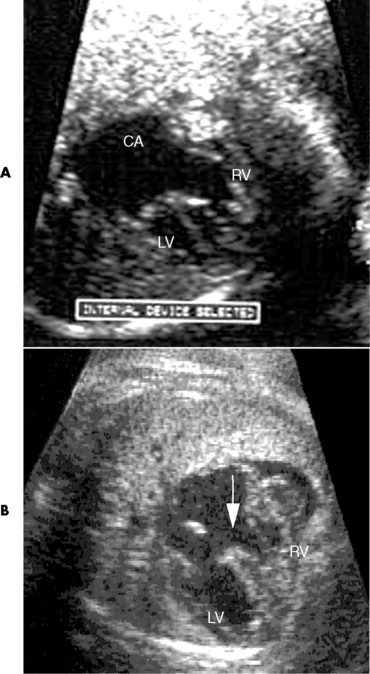
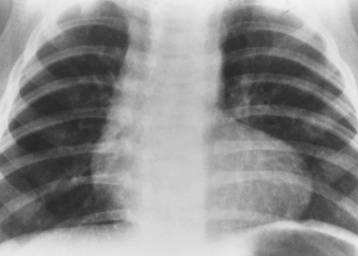
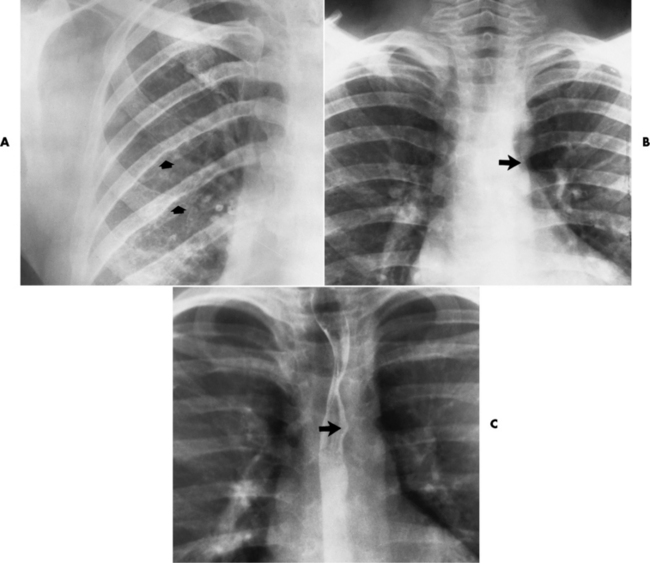
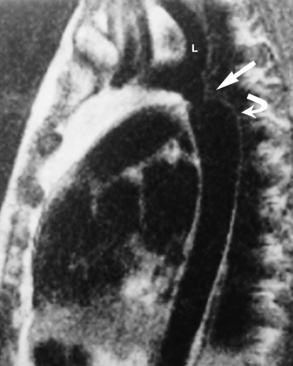
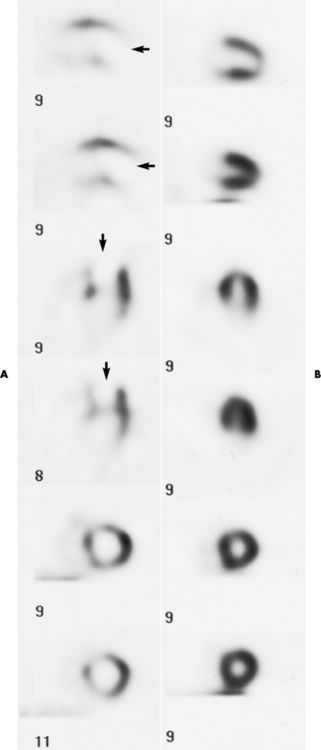
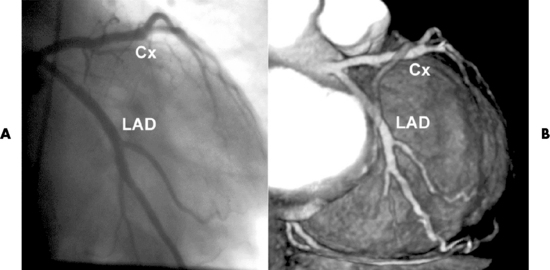
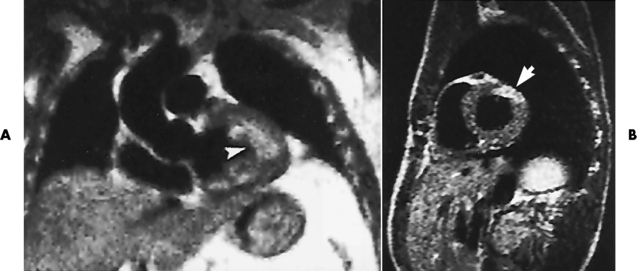
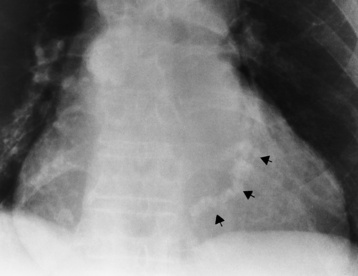
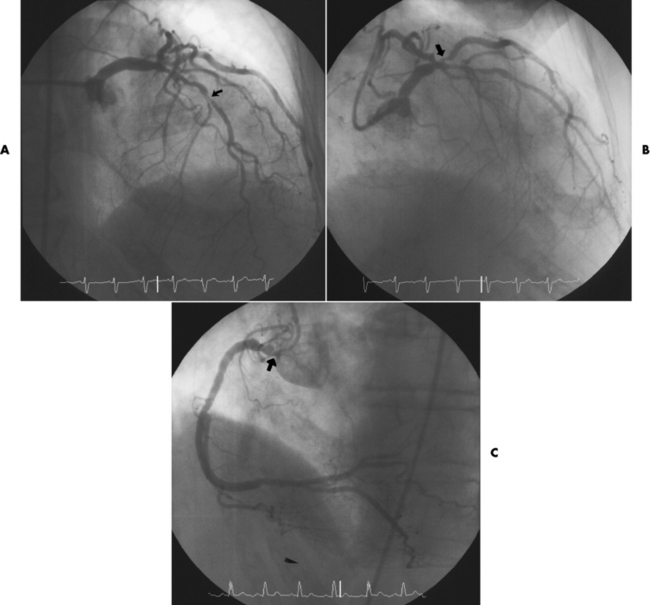
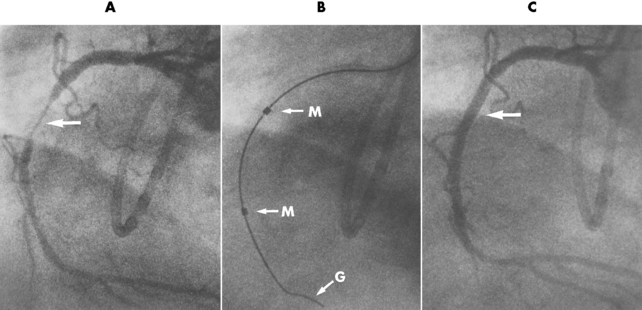
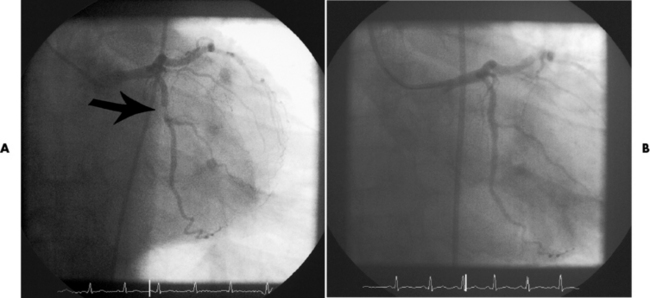
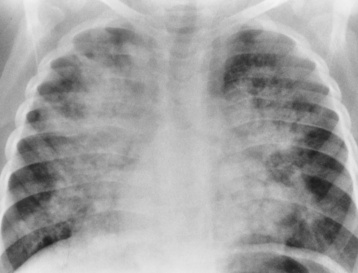
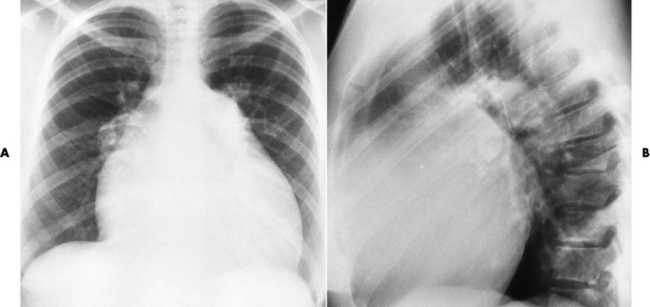
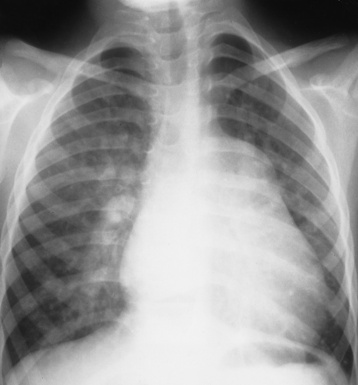
Chapter 7 After reading this chapter, the reader will be able to: 1 Define and describe all bold-faced terms in this chapter. 2 Describe the physiology of the cardiovascular system 3 Identify anatomic structures on both diagrams and radiographs of the cardiovascular system 4 Differentiate the various pathologic conditions affecting the cardiovascular system and their radiographic manifestations 5 Provide a description of the special procedures that are used when imaging particular pathologic conditions The heart consists of four chambers whose walls are composed of striated muscle (myocardium), and it is lined with a smooth delicate membrane (endocardium), which is continuous with the inner surface of the blood vessels (Figure 7-1). The heart consists of two atria and two ventricles, with a partition (the septum) separating the right and left sides of the heart. The ventricles are considerably larger and thicker walled than the atria because they have a substantially heavier pumping load. Between each atrium and its associated ventricle are the atrioventricular valves (one on the right and one on the left side of the heart), which permit blood to flow in only one direction (Figure 7-2). These valves consist of flaps (or cusps) of endocardium that are anchored to the papillary muscles of the ventricles by cordlike structures called the chordae tendineae. The mitral valve (bicuspid valve) (left atrioventricular) between the left atrium and the left ventricle has two cusps, whereas the tricuspid valve (right atrioventricular) between the right atrium and the right ventricle has three cusps. The semilunar valves separate the ventricles from the great vessels leaving the heart. The pulmonary valve lies between the right ventricle and the pulmonary artery, whereas the aortic valve separates the aorta from the left ventricle. Deoxygenated venous blood is returned to the heart from the body through the superior and inferior venae cavae, which empty into the right atrium. Blood flows from the right atrium across the tricuspid valve into the right ventricle, which then pumps blood through the pulmonary valve into the pulmonary artery. Within the capillaries of the lungs, the red blood cells take up oxygen and release carbon dioxide. The freshly oxygenated blood then passes through the pulmonary veins into the left atrium, from which it flows across the mitral valve into the left ventricle. Contraction of the left ventricle forces oxygenated blood through the aortic valve into the aorta and the rest of the arterial tree to provide oxygen and nourishment to tissues throughout the body. The general circulation of the body is termed the systemic circulation (Figure 7-3), whereas the circulation of blood through the lungs is the pulmonary circulation. Because greater pressure is needed to pump blood through the systemic circulation than through the pulmonary circulation, the wall of the left ventricle is considerably thicker than that of the right ventricle. The atria and ventricles alternately contract and relax. The contraction phase is called systole; the heart chambers relax and fill with blood during diastole (the relaxation phase). The normal cardiac impulse that stimulates mechanical contraction of the heart arises in the sinoatrial (SA) node, or pacemaker, which is situated in the right atrial wall near the opening of the superior vena cava. The impulse passes slowly through the atrioventricular (AV) node, which is located in the right atrium along the lower portion of the interatrial septum, and then spreads quickly throughout the ventricles by way of a band of atypical cardiac muscle fibers the bundle of His. The bundle of His terminates in Purkinje fibers, which can conduct impulses throughout the muscle of both ventricles and stimulate them to contract almost simultaneously. Specialized pacemaker cells in the SA node possess an intrinsic rhythm, so that even without any stimulation by the autonomic nervous system the node itself initiates impulses at regular intervals (about 60 to 75 beats per minute). If the SA node for some reason is unable to generate an impulse, pacemaker activity shifts to another excitable component of the conduction system. These ectopic pacemakers also generate impulses rhythmically, although at a much slower rate. For example, if the AV node were to control pacemaker activity, the heart would beat 40 to 60 times per minute. If the conduction system of the heart is unable to maintain an adequate rhythm, the patient may receive an artificial pacemaker that electrically stimulates the heart, either at a set rhythm or only when the heart rate decreases below a preset minimum. The heart is surrounded by a double membranous sac termed the pericardium. The pericardium has a well-lubricated lining that protects against friction and permits the heart to move easily during contraction. The most common congenital cardiac lesions are left-to-right shunts, which permit mixing of blood in the systemic and pulmonary circulations. Because blood is preferentially shunted from the high-pressure systemic circulation to the relatively low-pressure pulmonary circulation, the lungs become overloaded with blood. The magnitude of the shunt depends on the size of the defect and the differences in pressure on the two sides. An increased load on the heart produces enlargement of specific cardiac chambers, depending on the location of the shunt. The size of the defect determines the hemodynamic consequence for the systemic cardiac output. The most common congenital cardiac lesion is atrial septal defect, which permits free communication between the two atria as a result of either lack of closure of the foramen ovale after birth or its improper closure during gestation. Because the left atrial pressure is usually higher than the pressure in the right atrium, the resulting shunt is from left to right and causes increased pulmonary blood flow and overloading of the right ventricle. This produces the radiographic appearance of enlargement of the right ventricle, the right atrium, and the pulmonary outflow tract (Figure 7-4). Figure 7-4 Atrial septal defect. Frontal projection of the chest demonstrates cardiomegaly along with an increase in pulmonary vascularity reflecting a left-to-right shunt. Small aortic knob (white arrow) and descending aorta (small arrows) are dwarfed by enlarged pulmonary outflow tract (open arrow). In a patient with a ventricular septal defect, the resulting shunt is also from left to right because the left ventricular pressure is usually higher than the pressure in the right ventricle. The shunt causes increased pulmonary blood flow and consequently increased pulmonary venous return (Figure 7-5). This leads to diastolic overloading and enlargement of the left atrium and left ventricle. Because shunting occurs primarily in systole and any blood directed to the right ventricle immediately goes into the pulmonary artery, there is no overloading of the right ventricle and radiographically no right ventricular enlargement is seen. Figure 7-5 Ventricular septal defect. Heart is enlarged and somewhat triangular, and there is increased pulmonary vascular volume. Pulmonary trunk is very large and overshadows the normal-sized aorta, which seems small by comparison. The third major type of left-to-right shunt is patent ductus arteriosus. The ductus arteriosus is a vessel that extends from the bifurcation of the pulmonary artery to join the aorta just distal to the left subclavian artery. It serves to shunt blood from the pulmonary artery into the systemic circulation during intrauterine life. Persistence of the ductus arteriosus, which normally closes soon after birth, results in a left-to-right shunt. The flow of blood from the higher-pressure aorta to the lower-pressure pulmonary artery causes increased pulmonary blood flow, and an excess volume of blood is returned to the left atrium and left ventricle. Radiographically, there is enlargement of the left atrium, the left ventricle, and the central pulmonary arteries, along with a diffuse increase in pulmonary vascularity (Figure 7-6). The increased blood flow through the aorta proximal to the shunt produces a prominent aortic knob in contrast to the small- or normal-size aorta seen in atrial and ventricular septal defects. All left-to-right shunts can be complicated by the development of pulmonary hypertension (Eisenmenger’s syndrome). This is caused by increased vascular resistance within the pulmonary arteries related to chronic increased flow through the pulmonary circulation. Pulmonary hypertension appears radiographically as an increased fullness of the central pulmonary arteries with abrupt narrowing and pruning of peripheral vessels (Figure 7-7). The elevation of pulmonary arterial pressure tends to balance or even reverse the left-to-right shunt, thus easing the volume overloading of the heart. Doppler echocardiography can identify atrial and ventricular defects and extent of blood flow to determine the severity of the left-to-right shunting (Figure 7-8). Pressures can be measured to determine cardiac output from both the atria and ventricles. Dilatation of the ventricles implies patent ductus arteriosus. Tetralogy of Fallot is the most common cause of cyanotic congenital heart disease. It consists of four (thus “tetra”) abnormalities: (1) high ventricular septal defect, (2) pulmonary stenosis, (3) overriding of the aortic orifice above the ventricular defect, and (4) right ventricular hypertrophy. Pulmonary stenosis causes an elevation of pressure in the right ventricle and hypertrophy of that chamber. Because of the narrow opening of the pulmonary valve, an inadequate amount of blood reaches the lungs to be oxygenated. The ventricular septal defect and the overriding of the aorta produce right-to-left shunting of unoxygenated venous blood into the left ventricle and then into the systemic circulation, thus increasing the degree of cyanosis. Enlargement of the right ventricle causes upward and lateral displacement of the apex of the heart (Figure 7-9). This results in the classic coeur en sabot appearance, in which the heart resembles the curved-toe portion of a wooden shoe. In about one fourth of patients with tetralogy of Fallot, the aorta is on the right side. Coarctation of the aorta is often seen radiographically as rib notching (usually involving the posterior fourth to eighth ribs) resulting from pressure erosion by dilated and pulsating intercostal collateral vessels, which run along the inferior margins of these ribs (Figure 7-10A). Notching of the posterior border of the sternum may be produced by dilation of mammary artery collaterals. Coarctation of the aorta often causes two bulges in the region of the aortic knob that produce a characteristic figure-3 sign on plain chest radiographs (Figure 7-10B) and a reverse figure-3 (or figure-E) impression on the barium-filled esophagus (Figure 7-10C). The more cephalic bulge represents dilatation of the proximal aorta and the base of the subclavian artery (prestenotic dilatation); the lower bulge reflects poststenotic aortic dilatation. Aortography can accurately localize the site of obstruction, determine the length of the coarctation, and identify any associated cardiac malformations. More recently, MRI has been used to demonstrate aortic narrowing (Figure 7-11) and to evaluate the appearance of the aorta after corrective surgery. Occlusion of a coronary artery deprives an area of myocardium of its blood supply and leads to the death of muscle cells (myocardial infarction) in the area of vascular distribution. The size of the coronary artery that is occluded and the myocardium that it supplies determines the extent of heart muscle damage. The greater the area affected, the poorer the prognosis because of the increased loss of pumping function that may result in congestive heart failure (CHF). A favorable prognostic factor is the development of collateral circulation, through which blood from surrounding vessels is channeled into the damaged tissue. If the patient survives, the infarcted region heals with fibrosis. Long-term complications include the development of thrombi on the surface of the damaged area and the production of a local bulge (ventricular aneurysm) at the site of the weakness of the myocardial wall. Radionuclide CT scanning (single-photon emission computed tomography, or SPECT), using technetium pyrophosphate or other compounds that are taken up by acutely infarcted myocardium, is a new noninvasive technique for detecting, localizing, and classifying myocardial necrosis (Figure 7-12). Newer CT technology (spiral multidetector) provides visualization of the coronary arteries in a noninvasive approach. CT quantifies the artery calcification, evaluates stenosis, measures cardiac function, and analyzes plaque. To best demonstrate the coronary arteries, electrocardiography-synchronized scanning is completed in the diastolic phase (Figure 7-13, p. 260). On CT, areas of myocardial ischemia appear as regions of decreased attenuation because of the higher water content resulting from intramyocardial cellular edema. Multidetector helical CT scans provide calcium screening for detecting hard plaque. To evaluate soft plaque, CT angiography (CTA) is required. Studies have indicated that MRI is also of value in detecting early signs of muscular necrosis in myocardial infarction (Figure 7-14, p. 260) and may help determine whether the infarction is acute or remote. Plain chest radiographs are usually normal or nonspecific in most patients with ischemic heart disease. Calcification of a coronary artery, although infrequently visualized on routine chest radiographs and usually requiring cardiac fluoroscopy, strongly suggests the presence of hemodynamically significant coronary artery disease (Figure 7-15, p. 260). Plain chest radiographs are also entirely normal in many, if not most, patients after myocardial infarction. The images are primarily of value in detecting evidence of pulmonary venous congestion in patients in whom CHF develops as a result of an inability of the remaining heart muscle to propel blood through the circulation adequately. Coronary arteriography is generally considered the definitive test for determining the presence and assessing the severity of coronary artery disease. About 30% of significant stenoses involve a single vessel, most commonly the anterior descending artery. Another 30% involve two vessels, and significant stenosis of the three main vessels can be demonstrated in the remaining 40%. About 50% of coronary artery disease occurs in the left coronary artery, 35% in the right coronary artery, and 15% in the left circumflex artery (Figure 7-16, p. 261). Percutaneous transluminal coronary angioplasty (PTCA) using a balloon catheter is now a recognized procedure for the treatment of patients with narrowing of one or more coronary arteries (Figure 7-17, p. 262). As in other types of PTA, a catheter is placed under fluoroscopic guidance into the affected coronary artery, and an arteriogram is performed for localization. The angioplasty balloon is then positioned at the level of the stenosis and inflated. After dilation, coronary arteriography is repeated to illustrate the resulting appearance of the stenosis and to detect any complications of the procedure. Symptomatic improvement occurs in 50% to 70% of dilations. About 3% to 8% of patients who undergo PTCA experience either persistent coronary insufficiency or sudden occlusion of a coronary artery at the site of dilation at the time of the procedure. Therefore, the procedure should be performed at a time when an operating room, an anesthetist, and a cardiac surgeon are available so that immediate coronary bypass surgery can be performed if necessary. The percentage of deaths from coronary angioplasty is less than 1%. In conjunction with PTCA, deployment of a drug-eluting stent helps in many cases to maintain the open lumen (Figure 7-18, p. 262). Other interventional procedures are endovascular stenting, atherectomy, and laser-assisted angioplasty. Left-sided heart failure produces a classic radiographic appearance of cardiac enlargement, redistribution of pulmonary venous blood flow (enlarged superior pulmonary veins and decreased caliber of the veins draining the lower lungs), interstitial edema, alveolar edema (irregular, poorly defined patchy densities), and pleural effusions (Figure 7-19, p. 263). In acute left ventricular failure resulting from coronary thrombosis, however, there may be severe pulmonary congestion and edema with very little cardiac enlargement. Pulmonary congestion and edema may require a change in radiographic technique to compensate for the increased fluid in the lungs. The major causes of left-sided heart failure include coronary heart disease, valvular disease, and hypertension. In right-sided heart failure, dilatation of the right ventricle and right atrium is present (Figure 7-20, p. 263). The transmission of increased pressure may cause dilatation of the superior vena cava, widening of the right superior mediastinum, and edema of the lower extremities. The enlargement of a congested liver may elevate the right hemidiaphragm. Common causes of right-sided heart failure are pulmonary valvular stenosis, emphysema, and pulmonary hypertension resulting from pulmonary emboli.
Cardiovascular System
Physiology of the cardiovascular system
Congenital heart disease
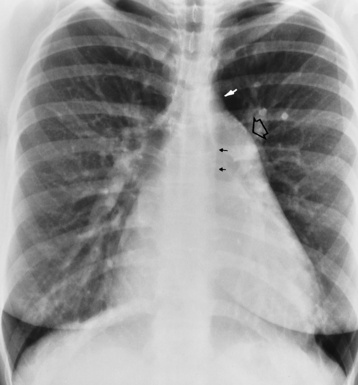
Radiographic Appearance
Tetralogy of Fallot
Radiographic Appearance
Coarctation of the Aorta
Radiographic Appearance
Acquired vascular disease
Radiographic Appearance
Treatment
Congestive Heart Failure
Radiographic Appearance
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Cardiovascular System