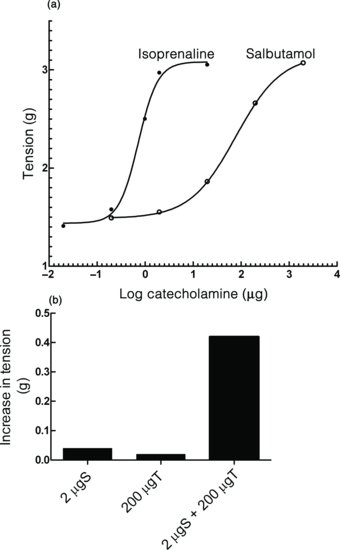
It should be seen that salbutamol is about 100 times less potent than isoprenaline, suggesting that both drugs were acting on β1-adrenoceptors in the heart. At the doses selected, neither salbutamol nor theophylline produce responses, but together they produce a clear response.
Questions
Figure 5.2 (a) Dose–response curves for isoprenaline and salbutamol in a Langendorff preparation of a guinea pig heart. The relative potencies of the two agonists can be measured from the third graph. (b) Bar graphs of the responses to salbutamol, theophylline and theophylline and salbutamol together in the guinea pig Langendorff heart preparation.
5.1.3 The Rat Isolated Auricle Preparation
This preparation is adapted from that described by Saunders and Thornhill (1985). An isometric transducer is connected to a bridge amplifier and an ADInstruments PowerLab amplifier and AD converter. Responses are recorded by a computer running Chart software.
Experimental Conditions
| Organ bath | 20 mL |
| Ringer | Krebs |
| Aeration | 95% CO2/5% O2 |
| Temperature | 37°C |
| Transducer | Isometric |
| Resting tension | 8 g |
| Dosing | Obtain cumulative concentration–response curves as the preparation may take 5–10 min to recover a steady rate after washing. |
| Electrical stimulation | |
| Voltage | 5 V above maximal |
| Pulse duration | 20 ms |
| Frequency | 6 Hz (mimics resting heart rate of a rat, 360 bpm) |
The heart is rapidly removed from a guinea pig and placed in Locke–Ringer solution which has been cooled to 4°C in an ice bath. This stops the beating of the heart and minimizes damage to heart tissue due to ischaemia during the dissection of the heart. The ventricles are cut away and surrounding tissue removed until all that remains are the left and right auricles. The auricles are appendages of the atria. A cotton thread is tied around the tip of each auricle, one of which is anchored to a metal support and the other left free to attach to the transducer. The tissue is then mounted in an organ bath and the thread is attached to the transducer. When the tissue warms up to 37°C it will start to beat again, and should be allowed to equilibrate when the rhythmic beating becomes regular.
A concentration–response curve can be obtained at organ bath concentrations between 10−8 M and 5 × 10−6 M of noradrenaline. The presence of β1-adrenoceptors can be demonstrated by obtaining a concentration–response curve with isoprenaline (a non-selective β-adrenoceptor agonist) between similar concentrations. This should be inhibited by 10−6 M atenolol (a β1-selective adrenoceptor antagonist).
5.2 THORACIC AORTA PREPARATION
It is generally thought that the innervation of blood vessels is mainly by the sympathetic division of the autonomic nervous system. In the aorta, adrenoceptors mediating constriction are mainly of the α1 sub-type. β2-receptors mediate dilation, but mainly in skin and peripheral tissues. Only some blood vessels have parasympathetic innervation, but acetylcholine causes vasodilation in vivo. In isolated aortic strip preparations, acetylcholine was notorious for producing erratic responses, sometimes causing contraction, sometimes dilation. This problem was resolved in the seminal report by Furchgott and Zawadzki (1980), who found that if great care was taken not to damage the inner endothelial lining of the aorta, acetylcholine reproducibly produced relaxation. A similar finding is found for some other vasodilators, such as bradykinin and substance P. The isolated aortic strip preparation was instrumental in elucidating the essential role of the endothelium in controlling vascular tone. The factor released by endothelial cells which subsequently acted on vascular smooth muscle was termed endothelium-derived relaxing factor (EDRF). Subsequently, it was demonstrated that this relaxing factor was in fact the unstable free radical, nitric oxide (reviewed by Furchgott, 1998 in his Nobel lecture). Some vasodilators are not endothelium dependent, and a number of them are used in the treatment of hypertension. Glyceryl trinitrate (and other nitric oxide donors) act directly on (more accurately in) smooth muscle cells to release nitric oxide which activates guanylate cyclase. Other vasodilators, such as calcium channel antagonists (e.g. nifedipine), act by antagonizing L-type calcium channels (see Section 4.2.6).
Experimental Conditions
| Organ bath volume | 20 mL |
| Ringer solution | Krebs |
| Aeration | 95% O2/5% CO2 |
| Bath temperature | 37°C |
| Transducer | Isometric |
| Resting tension | 2 g |
| Contact time | 3–5 min |
| Dose cycle | Obtain cumulative curves. Allow 5–10 min to relax. |
Method
A short section is carefully removed from the rat and dissected free from connective tissue and fat. Utmost care must be taken in handling the aorta section so as not to damage the interior endothelial lining of the vessel. 1.5–2 cm segments of rat aorta are mounted horizontally in a water-jacketed organ bath of 10 mL filled with Krebs–Heinseleit solution (see Section 2.3) maintained at 37°C. The solution is aerated with a gas mixture containing 95% O2:5% CO2. The rings are suspended horizontally on a pair of stainless steel hooks, one of which was fixed to an L-shaped rod inside the chamber and the other to an isometric transducer (PowerLab ML750). The stainless steel hook is connected to the force displacement transducer. Isometric contractions are measured and recorded continuously in a computer by using the Chart software. Arterial rings are equilibrated in Krebs–Henseleit solution for 1 h at 1 g optimum resting force. In some rings, the endothelium can be removed gently by rubbing the luminal surface of the ring with a roughened polyethylene tube. At the end of the equilibration period, viabilities of the arterial segments with and without endothelium are checked by depolarization with KC1 (60 mM) and phenylephrine (10−5 M). The response of this tissue is relatively slow, and it will take 5 min or more to reach full contraction. Cumulative dose–response curves are therefore routinely employed. The effectiveness of endothelium removal is confirmed by the inability of acetylcholine (10−6 M) to induce relaxation of phenylephrine-precontracted rubbed rings when less than 10% of maximum relaxation should be obtained.
5.2.1 Drugs Regulating Nitric Oxide-mediated Relaxation
This experiment is intended to demonstrate that some vasodilators are dependent on the presence of an intact endothelium and others act independently of the endothelium. Endothelium-dependent vasodilators, such as acetylcholine and bradykinin, act via a receptor on the endothelium membrane. This results in a rise in endothelial intracellular [Ca2+], which activates the enzyme endothelial nitric oxide synthase (eNOS). Nitric oxide produced diffuses into adjacent smooth muscle cells where it activates soluble guanylate cyclase. The increase in cGMP results in a decrease in intracellular [Ca2+] in smooth muscle cells. Here, acetylcholine is used as an endothelium-dependent vasodilator. The idea that acetylcholine-stimulated vasodilation requires activation of eNOS is demonstrated by inhibiting this enzyme with Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME). If a source of NO is supplied exogenously in the form of a NO donor, such as sodium nitroprusside (SNP) or glyceryl trinitrate, the vasodilation can be restored.
Procedure
The following stock concentrations of drugs should be available: 2 mM phenylephrine containing few milligrams of ascorbic acid, 2 mM acetylcholine, 10 mM L-NAME and 10 mM and 10 μM SNP (protected from light).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


