Cardiovascular Pathology
EMBRYOLOGY
What is the ductus arteriosus?
It connects the pulmonary artery to the proximal aorta, effectively by passing the lungs during fetal development.
What happens if the ductus doesn’t close after birth?
If it remains patent after birth, the neonate becomes hypoxic (patent ductus arteriosus, PDA).
The sinus venosus gives rise to which parts of the cardiovascular system?
A portion of the wall of the right atrium and the coronary sinus
The bulbus cordis gives rise to which parts of the cardiovascular system?
The proximal aorta and the pulmonary arteries
What is the function of the ductus venosus?
To provide a direct passageway for nutrient-rich blood from the placenta to pass through the developing liver to supply the developing heart
What happens to the ductus venosus at birth?
It becomes obliterated and fibrosed, forming the ligamentum venosum.
ANATOMY
Define the anatomic components of the cardiovascular system:
Heart, macrovasculature (aorta, arteries, large arterioles, veins), microvasculature (small arterioles, postcapillary venules, capillaries), and lymphatics
Name the four heart valves in the direction of blood flow:
- Tricuspid valve
- Pulmonary valve
- Mitral valve
- Aortic valve
What structure provides the base of attachment for the cardiac valves?
The so-called “fibrous skeleton” of the heart, which is composed of dense connective tissue and has three main components: the septum membranaceum, the trigona fibrosa, and the annuli fibrosi. The base of each cardiac valve is attached to the annuli fibrosi.
What is the anatomic location of the carotid bodies and what is their function?
The carotid bodies are located near the bifurcation of the common carotid artery (bilaterally), and they function as chemoreceptors monitoring levels of carbon dioxide and oxygen in the blood.
What is the anatomic location of the carotid sinus and what is their function?
The carotid sinuses are dilatated segments of the internal carotid artery (bilaterally), and they contain baroreceptors which detect changes in blood pressure and transmit this information to the central nervous system.
At what anatomic location does lymphatic fluid reenter the bloodstream?
Either via the thoracic duct at the confluence of the left internal jugular and left subclavian veins or via the right lymphatic duct at the confluence of the right internal jugular and right subclavian veins.
Which two organs do not have lymphatic drainage?
- Central nervous system
- The bone marrow
HISTOLOGY
Name the three microscopically identifiable “coats” of blood vessel walls:
- Tunica (L. “coat”) intima
- Tunica media
- Tunica adventitia
Which additional named structures of blood vessel walls are identifiable in arteries?
Internal elastic lamina (separates the tunica intima from the tunica media) and the external elastic lamina (separates the tunica media from the tunica adventitia)
What is the cellular composition of the tunica intima?
A single layer of endothelial cells supported by a subendothelial layer of loose connective tissue with scattered smooth muscle cells.
Of what are the internal and external elastic lamina composed? What are the functions of these structures?
Elastin. Gaps in the internal elastic lamina (aka fenestrae) allow nutritive substances from the blood to diffuse to cells located deeper in the vessel wall. The elastic properties of both the internal and external lamina serve to modulate the degree of pressure variation in vessels during systole and diastole.
What is the cellular and extracellular composition of the tunica media?
Concentric layers of smooth muscle cells admixed with variable amounts of elastic fibers, type III collagen, proteoglycans, and glycoproteins produced by the smooth muscle cells
What is unique about the tunica media of the carotid sinus?
It is thinner than the tunica media in other vessels which allows baroreceptors in this segment of the internal carotid artery to detect changes in blood pressure and transmit this information to the central nervous system.
What is the composition of the tunica adventitia?
Type I collagen and elastic fibers
What are vasa vasorum?
Arteries, capillaries, and venules in the tunica adventitia and outer tunica media of larger vessels that provide nutrients to these outer layers
Describe the innervation of blood vessels:
In vessels containing smooth muscle cells, sympathetic nerve fibers discharge norepinephrine to cause vasoconstriction. Vessels that supply blood to skeletal muscles also have cholinergic innervation to cause vasodilation. Density of innervations is higher in arteries than in veins.
Which histologic layer of the wall of the heart is homologous with the tunica intima of blood vessels?
Endocardium
Where do branches of the Purkinje system terminate within the heart?
In the subendocardial layer, which also contains veins and other nerve branches
What cells compose the myocardium and to what fibrous structure are they associated?
The myocardium is composed of cardiac muscle cells (aka cardiomyocytes). These cells are arranged in layers which form a complex spiral around the chambers of the heart. Many cardiomyocytes are anchored to the fibrous cardiac skeleton.
PHYSIOLOGY
What is cardiac output (CO)?
The volume of blood pumped by the heart per unit time. CO (mL/minute) = stroke volume (mL/beat) × heart rate (beats/minute)
What is ejection fraction (EF)?
Of the volume of blood present in the left ventricle at the end of diastole (EDV), EF is the percentage of that volume that is pumped per beat. Normal EF ≥ 55%. EF = (SV/EDV) × 100
Changes in which variables will affect cardiac output?
Stroke volume and heart rate
Changes in which variables will affect stroke volume?
Preload, afterload, and contractility
At which phase of the myocardial action potential does calcium enter the cardiomyocytes?
Phase 2 (plateau)—calcium enters via voltage-gated calcium channels
What is the consequence of calcium influx on cardiomyocyte contraction?
Calcium influx triggers additional calcium release from the sarcoplasmic reticulum. Calcium binds troponin C inducing a conformational change in troponin I and movement of the troponin-tropomyosin complex out of the actin filament active site. When this active site is bound by myosin, cross-bridges form and contraction can occur.
What is the function of the three cardiac troponins?
- Troponin C binds calcium and is bound to both troponin T and troponin I
- Troponin T is bound to tropomyosin
- Troponin I is bound to actin and holds the troponin-tropomyosin complex in place
How is the smooth muscle contraction apparatus different from the cardiac muscle contraction apparatus?
Smooth muscle contraction is dependent on calcium binding calmodulin to activate myosin light chain kinase and phosphorylate myosin leading to cross-bridge formation and contraction. Smooth muscle contraction does not involve troponins.
Which phase of the pacemaker action potential undergoes diastolic depolarization?
Phase 4—the membrane potential will spontaneously depolarize as sodium conductance is increased, this accounts for the automaticity of the sinoatrial (SA) and atrioventricular (AV) nodes and subsequently for heart rate
PATHOLOGY
General Principles
What is the leading cause of death in the United States?
Heart disease
What entities are included in the category “heart disease”?
Coronary artery disease (CAD), cardiomyopathy, ischemic heart disease, hypertension, valvular disease, heart failure, and inflammatory heart disease
What other now common chronic condition increases a patient’s risk of experiencing a cardiovascular event?
Diabetes mellitus
What modifiable risk factors can increase a patient’s risk of experiencing a cardiovascular event?
Smoking, sedentary lifestyle, obesity, and hyperlipidemia
What is hyperlipidemia?
A state of having elevated quantities of lipid substances—cholesterol and triglycerides, in the blood
What is a clinical sign associated with hyperlipidemia?
Xanthomas—yellow to white waxy deposits commonly involving skin of the eyelids or Achilles tendon
How do xanthomas appear microscopically?
Diffuse dermatitis consisting of foamy histiocytes (lipophages)
Which laboratory tests may be used to assess for myocardial infarction?
Troponin and/or CK-MB are used to assess for cardiac infarction. Historically, myoglobin, LDH, AST levels were also used.
When do cardiac troponin levels increase after a myocardial infarction and how long will the levels remain elevated?
Levels will rise approximately 3 to 6 hours after infarction and may remain elevated for as long as 14 days after the event. (Note—there is a latent period, therefore if a patient presents with very acute infarction, the troponin level may initially not be elevated.)
Which laboratory test is used in diagnosis and management of congestive heart failure?
B-type natriuretic peptide (BNP)
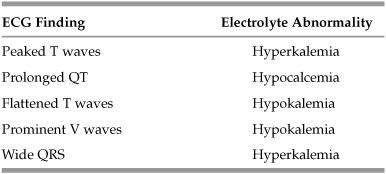
Table 6.1 EGG Findings and Electrolyte Abnormalities
Vascular
What are the risk factors for hypertension?
Smoking; Obesity; Diabetes; African American race/A ge
*SODA
What is the most common identifiable etiology of hypertension (HTN)?
Renal disease, which accounts for less than 10% of all cases of HTN; the remaining 90% of cases are termed “essential (primary) hypertension” and the underlying cause is not well-characterized.
What are the complications of uncontrolled HTN over time?
Aortic dissection; coronary heart disease; congestive heart failure (CHF); renal failure; stroke
What part of the brain parenchyma does uncontrolled hypertension affect first?
Basal ganglia and internal capsule
What is the difference between hypertensive urgency and emergency?
Urgency is only high blood pressure (>200/>120), whereas emergency is high blood pressure and end-organ damage.
What is atherosclerosis?
A process of thickening of the wall of any sized artery as a result of deposition of fatty materials (eg, cholesterol) and subsequent chronic inflammatory response
What are the risk factors associated with atherosclerosis?
Smoking; hypertension; hyperlipidemia; diabetes mellitus
What is the earliest histologic and/or gross finding associated with atherosclerosis?
Fatty streaks in a vessel walls
What do fatty streaks progress into?
Plaques—a nodular accumulation of fatty materials and macrophages which may be associated with cholesterol crystals and calcification
What is the most common arterial location of atherosclerosis?
Abdominal aorta
What is arteriosclerosis? Arteriolosclerosis?
- Arteriosclerosis is a term used to describe “hardening” of medium to large arteries.
- Arteriolosclerosis is a term used to describe “hardening” of small arteries.
*Note neither of these terms is specific for changes to artery walls due to atherosclerosis.
Define stable angina:
A clinical term used to describe chest pain that develops with exertion or stress and is relieved with rest
Define acute coronary syndrome (ACS):
A term used to describe a clinical presentation which may represent manifestations of one of several underlying pathologic processes. Generally, patients present with chest pain starting a rest or with minimal exertion that is not relieved with rest or nitroglycerine. ACS may represent unstable angina, ST-elevation myocardial infarction (STEMI), or non-ST-elevation MI (NSTEMI).
What is the difference between unstable angina and STEMI/NSTEMI?
In unstable angina, heart muscle is not damaged. In STEMI and NSTEMI, heart muscle undergoes ischemic damage and becomes infarcted.
What is the cause of stable angina?
Stable angina is a clinical scenario that can be caused by decreased blood flow to myocardium (eg, due to narrowing of vessel lumen by atherosclerosis), resistance of vasculature to blood flow, and decreased oxygen-carrying capacity of the blood.
Which drug relieves the chest pain associated with stable angina?
Usually nitroglycerin or vasodilators (ie, calcium channel blockers). If there is decreased oxygen-carrying capacity in the blood, the patient may need blood transfusion or other therapies.
What is the cause of unstable angina?
Atherosclerotic plaque disruption with subsequent platelet plug formation, possibly leading to thrombosis of a coronary vessel
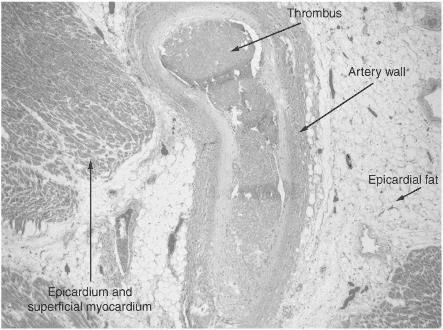
Figure 6.1 Thrombus in a coronary artery. (Reproduced, with permission, from OHSU.)
What is the cause of Prinzmetal angina?
Vasospasm that causes a clinically significant narrowing of the coronary vessels; the etiology of vasospasm is not known.
Which artery is the most commonly affected during acute MI?
Left anterior descending (LAD)
*Older LADs usually have Mis
How is an MI diagnosed?
Clinical history and depending on timing of presentation, cardiac enzymes, and abnormal ECG findings
What are the two patterns of MI?
- Transmural
- Subendocardial
What areas of the heart are affected with transmural infarctions?
Blood flow to the entire ventricular wall is compromised. Ultimately, necrosis extends from epicardium to endocardium.
What are the typical findings on ECG with transmural infarction?
ST segment elevation or Q waves
What areas of the heart are affected with subendocardial infarctions?
Only the inner one-third of usually the left ventricle wall
What are the ECG findings in acute subendocardial infarcts?
Nonspecific ischemic changes, ST depression
What are the earliest histologic changes associated with infarction?
Early features of coagulative necrosis with blurring of nuclear and cell borders
In general, what are the gross and microscopic changes observable in MI at autopsy?
In general, observable changes will vary depending on amount of elapsed time between infarction and autopsy. Gross changes can include: pallor or hyperemia, necrotic areas, early scar formation, and old scars from previous MI. Microscopic changes can include: blurring of cardiomyocyte nuclei and striations, neutrophils, macrophages, fibrosis, and scar formation.
During healing and repair, what type of necrosis does infracted myocardium undergo?
Coagulative necrosis
When are the first microscopic changes of coagulative necrosis in MI observable?
After 12 hours
What are the first cells to appear in the damaged tissue about 12 hours post-MI?
Neutrophils
*Neutrophils go to a New site of injury at Noon (8-12 hours after injury)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


