12 Cardiovascular disease
Cardiac arrhythmias
Approach to the patient
History
Examination
Investigations
Twelve-lead electrocardiogram (ECG)
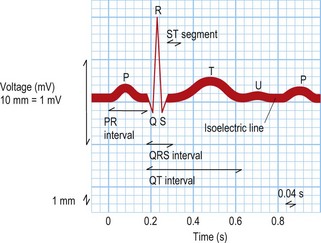
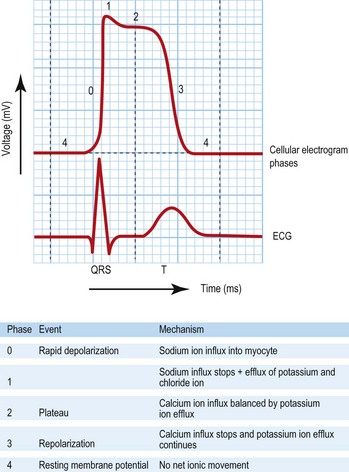
This test provides a three-dimensional snapshot of the electrical activity of the heart and is a useful screening tool. The sum of the depolarization (activation) and repolarization (recovery) potentials of the atrial and ventricular myocardium gives rise to the ECG waveform (Fig. 12.1).
Atrial depolarization spreads from the sinus node inferiorly and to the left, producing a P wave that is usually positive in lead II and negative in aVR. Ventricular activation begins with depolarization of the interventricular septum from left to right, producing a small, positive R wave in lead V1 and a small, negative Q wave in lead V6. Depolarization of the remaining ventricular mass is usually dominated by the more massive left ventricle and therefore directed to the left and posteriorly. This results in a negative S wave in lead V1 and a positive R wave in lead V6. The normal transition from leads V1 to V6 is marked by a progressive increase in R wave amplitude and a simultaneous decrease in S wave amplitude. QRS duration is usually 100 ms or less. Ventricular repolarization usually proceeds in the reverse direction to depolarization, i.e. apex to base and epicardium to endocardium, producing a deflection of similar polarity called the T wave. See Fig. 12.1 and Box 12.1.
Box 12.1 Normal ECG intervals
| P wave duration | 120 ms or less |
| PR interval | 120–200 ms |
| QRS duration | 100 ms or less |
| Corrected QT interval | 440 ms or less in males |
| 460 ms or less in females |
Electrophysiology study (EPS)
Table 12.1 The basic electrophysiology study
| Action | Measurements | Note |
|---|---|---|
| Sinus rhythm | Basic intervals (PA, AH, His duration, HV, QRS and corrected QT) | Is there pre-excitation or evidence for slowed conduction? |
| Single ventricular extra-stimulus testing | Retrograde AV node effective refractory period (AVNERP) | Is there VA conduction? If so, is it via the AV node and decremental, or an accessory pathway? |
| Incremental ventricular pacing | Retrograde Wenckebach cycle length (WCL) | |
| Single atrial extra-stimulus testing | Anterograde AVNERP | Is there AV conduction? If so, is it via the AV node and decremental, or an accessory pathway? Is there evidence for dual AV node physiology? |
| Incremental atrial pacing | Anterograde WCL | |
| Arrhythmia induction pacing from the atrium | Atrial effective refractory period (AERP) Coupling interval(s) for arrhythmia induction | Extra-stimulus testing with 2 or more extra-stimuli, burst pacing and 2 or more premature beats during sinus rhythm |
| Wellen’s protocol | Ventricular effective refractory period (VERP) Coupling interval(s) for arrhythmia induction | Used to induce ventricular tachycardia. Ventricular extra-stimulus testing with up to 3 extra-stimuli |
Pathophysiology of arrhythmias
Mechanisms and diagnosis of bradyarrhythmias
Sinus node-dependent arrhythmias
These include sinus bradycardia and sinus pauses with or without an escape rhythm.
Sinus bradycardia
Treatment of sinus bradycardia involves identifying and excluding specific causes, if possible. Specific measures are summarized in Box 12.2.
Atrio-ventricular node-dependent arrhythmias
Second-degree block
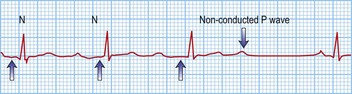
Third-degree or complete block (AV dissociation)
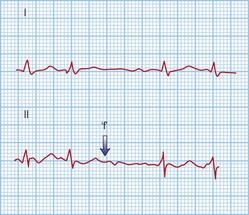
This is when no atrial electrical impulses are conducted to the ventricles. Hence, there is a continuously changing relationship between the P waves and QRS complexes (Fig. 12.4). Usually, there is a regular escape rhythm that arises below the level of block. A narrow QRS complex (< 125 ms) escape rhythm is from the His bundle and more stable than a broad QRS complex (> 125 ms), ventricular escape rhythm.
Bundle branch blocks and hemiblocks
These are due to delayed conduction within the His–Purkinje system, producing QRS complex durations > 120 ms. Causes include ischaemic heart disease, idiopathic fibrosis of the conduction system, cardiomyopathies, aortic stenosis, hypertensive heart disease, recurrent pulmonary emboli, cor pulmonale, congenital lesions, infective endocarditis and myotonic dystrophy.
Mechanisms and diagnosis of tachyarrhythmias
Atrial origin
Focal tachycardias
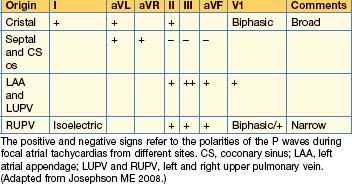
The morphology of the P waves during tachycardia is highly variable, depending on the site of the tachycardia focus. If the focus is close to the sinus node, typical P waves may be seen. P wave morphologies may give some indication as to the site of the tachycardia focus (Table 12.2).
Flutters
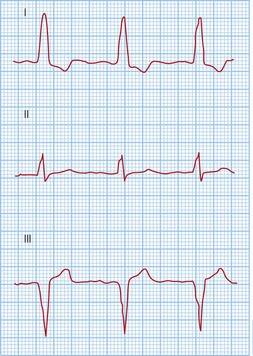
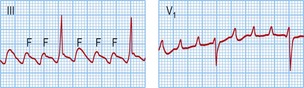
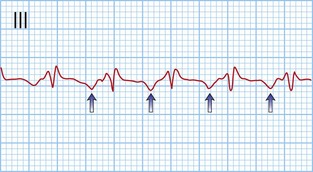
Typical atrial flutter usually, but not always, has negative sawtooth-like flutter waves in the inferior ECG leads and positive flutter waves in lead V1 (Fig. 12.6). Frequently, typical atrial flutter is conducted with a 2-to-1 AV block, giving a heart rate of 150 bpm. The re-entry circuit is well characterized, and the zone of slow conduction critical to sustaining the circuit is the cavo-tricuspid isthmus.
Atrial fibrillation (AF)
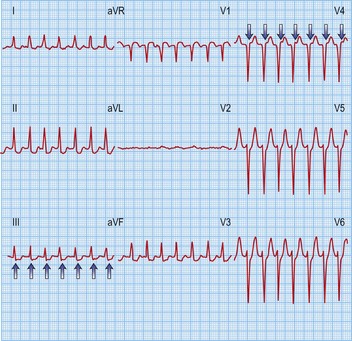
AF is the commonest arrhythmia globally, with a prevalence of 0.5–1% of the general population and 5–10% of the population over 65 years. It is characterized by rapid (300–600 bpm), irregularly irregular contractions of the atria. The 12-lead ECG shows no regular atrial activity (Fig. 12.7) and the ventricles beat in an irregularly irregular fashion at rates of up to 150–200 bpm. The mechanisms underlying AF are thought to be a combination of triggering atrial ectopics usually originating within the pulmonary veins, and progressive electrical changes within the atrial myocardium (remodelling) that allow it to be sustained. AF is a consequence of a wide range of pathologies but it can exist independently in a structurally normal heart (lone AF).
• Acute AF
• Chronic AF
Atrio-ventricular junction origin
Atrio-ventricular node re-entry tachycardia (AVNRT)
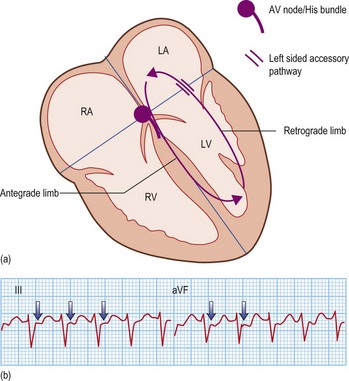
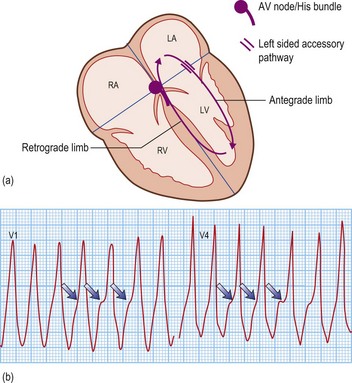
Accessory pathway-dependent tachycardias
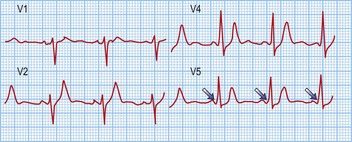
Long RP tachycardias
This is a special group of supraventricular tachycardias whose RP interval is > 50% of the RR interval (Fig. 12.14). The possible causes include focal atrial tachycardia, atypical AVNRT and orthodromic AVRT.
Ventricular origin
Ventricular tachycardia (VT)
Table 12.3 ECG features distinguishing ventricular tachycardia from supraventricular tachycardia with aberrant conduction
| ECG criterion | VT | SVT with aberrant conduction |
|---|---|---|
| AV relationship | Usually dissociated | Usually associated |
| Capture and fusion beats | Present | Absent |
| QRS duration | > 140 msec with RBBB morphology > 160 msec with LBBB morphology | ≤ 140 msec ≤ 160 msec |
| Mean frontal plane axis | −90 to ±180° | Similar to sinus rhythm |
| QRS transition across chest leads | Usually positive or negative concordance, i.e. no transition | Similar to sinus rhythm |
LBBB/RBBB, left/right bundle branch block.
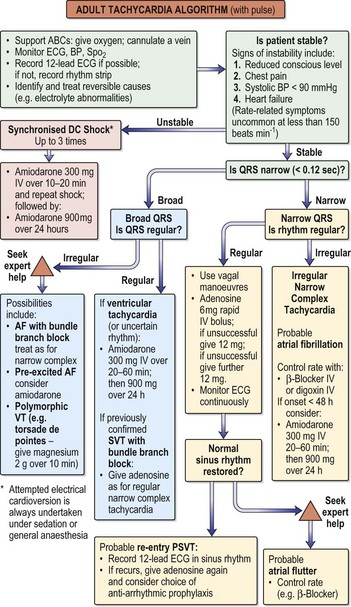
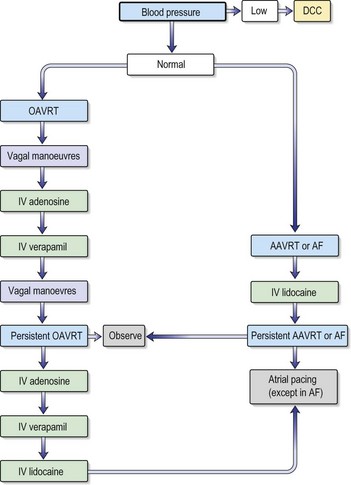
Fig. 12.15 Adult tachycardia algorithm (with pulse).
(Reproduced with permission from the Resuscitation Council (http://www.resus.org.uk/siteindex.htm.)
•Investigations
•Treatment
Ventricular fibrillation (VF)
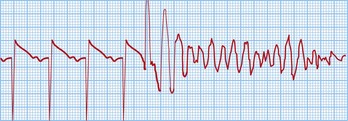
This is a cardiac arrest rhythm requiring immediate electrical defibrillation. There are advanced life support treatment algorithms that should be applied (p. 705). The underlying causes are similar to those of VT; the commonest is ischaemic heart disease with previous or acute myocardial infarction. The ECG shows disorganized electrical activity (Fig. 12.16) that is usually sustained. Unless there is an obvious reversible cause, such as acute myocardial ischaemia, all patients require ICD implantation.
Sudden cardiac death
Ion channel disorders
Long QT syndrome (LQTS)
This is another cause of potentially lethal ventricular arrhythmias in structurally normal hearts and presents with sudden death or syncope. It is characterized by prolongation of the QT interval > 440 msec due to abnormal myocardial repolarization. The underlying causes may be ion channel mutations, certain drugs and electrolyte disturbances. The prevalence is around 1 in 4000, although it is probably under-diagnosed.
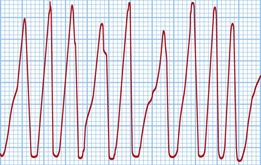
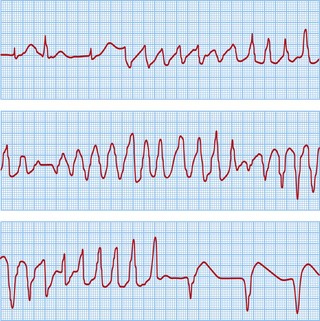
The classical VT in LQTS is torsades de pointes. This is a polymorphic VT in which the amplitude of the QRS complexes constantly changes around the isoelectric line (Fig. 12.17). This causes palpitations or syncope. Most episodes are self-terminating but SCD results from degeneration into VF.
Drug therapy
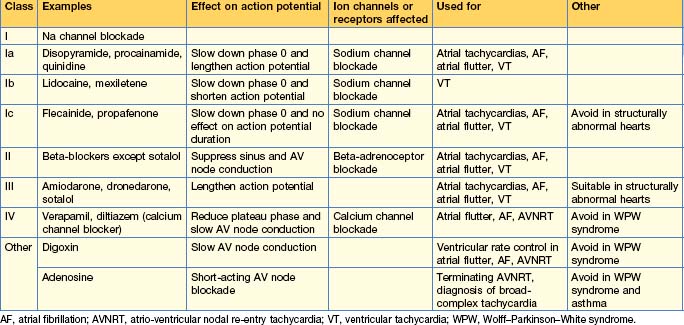
Anti-arrhythmic drugs are commonly used and work by affecting different components of the cardiac action potential. Consequently, they may precipitate arrhythmias and depress cardiac contractility. The Vaughan Williams classification describes them according to the effect on the action potential (Table 12.4). The use of individual drugs has been outlined in previous sections.
Device therapy
Pacing
Table 12.5 International pacemaker nomenclature
| I Chamber paced | II Chamber sensed | III Response to sensing |
|---|---|---|
| O: None | O: None | O: None |
| A: Atrium | A: Atrium | T: Triggered |
| V: Ventricle | V: Ventricle | I: Inhibited |
| D: Dual (A and V) | D: Dual (A and V) | D: Dual (T and I) |
DDD and VVI are most commonly used.