22 Cardiothoracic surgery
Basic considerations
Pathophysiological assessment
Careful history and appropriate examination suggest the presence of possible cardiac pathology. The initial clinical assessment is then refined and specific investigations used to confirm and quantify any disease identified (Table 22.1).
Table 22.1 Specific assessments of cardiac pathophysiological status
| Investigation | Yield |
|---|---|
| ECG | |
| Resting | Rhythm; conduction abnormalities; atrial and ventricular hypertrophy; established ischaemic changes; evidence of previous myocardial infarction |
| Exercise | Exercise-induced ischaemic changes or arrhythmias |
| Chest X-ray | Cardiac enlargement; valvular calcification; evidence of pulmonary oedema (Kerley B lines, pleural effusion, interstitial marking, hilar flare); absent or enlarged cardiac or great vessel structures |
| Thallium isotope scan | Areas of low radio-uptake indicative of impaired myocardial perfusion |
| Echocardiography | |
| Precordial | Ventricular contractility; valvular stenoses, regurgitation or leaflet abnormalities; intracardiac morphology, including septal defects and intracardiac masses; pericardial effusion |
| Transoesophageal | Enhanced views of posterior cardiac structures (aortic and mitral valves, ascending aorta, great veins and posterior septae); posterior pericardial fluid collections |
| Cardiac catheterization | |
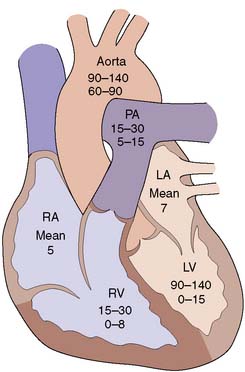
| Chamber pressures | Assess left and right ventricular function via determination of left ventricular end-diastolic pressure; atrial pressures in valve disease; transvalvular gradients (Fig. 22.1) |
| Angiography | Coronary arterial anatomy; intracardiac anatomy; trans-septal flow |
| O2 saturations | Intracardiac shunts |
| Cardiac output | Cardiac function and determination of secondary derived parameters, including peripheral and pulmonary vascular resistance |
Assessment of risk
Stroke
Stroke risk varies from 1% to over 10%, and is associated with intracardiac thrombus and severe atheromatous disease of the proximal aorta and carotids. Patients with evidence of peripheral vascular disease have a higher risk of stroke and those with high-grade symptomatic carotid disease may benefit from carotid endarterectomy prior to cardiac surgery (Ch. 21).
Specific aspects of surgical technique
Cardiopulmonary bypass (CPB)
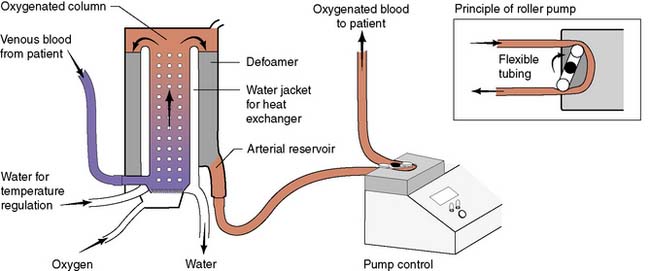
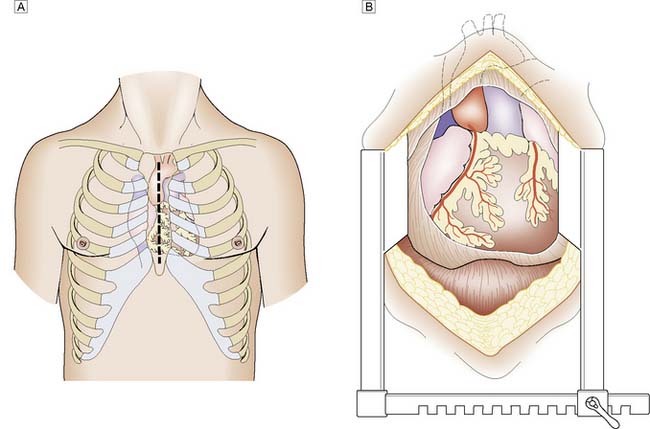
Modern cardiac and great vessel surgery became feasible with the development of cardiopulmonary bypass. Venous blood is drained via cannulae inserted into the right atrium or venae cavae and passes to a reservoir. It is then pumped through an oxygenator, which adds O2 and removes CO2, through a heat exchanger coil so that its temperature can be varied and finally, the blood is returned to the arterial circulation via a cannula in the ascending aorta or other suitable artery (femoral, axillary) (Figs 22.1, 22.2 and 22.3). Full anticoagulation with intravenous heparin is required to prevent blood clotting in the tubing, oxygenator and pump mechanisms. Roller or centrifugal pumps are used, as these minimize red cell trauma. Semipermeable membranes, or more commonly hollow fibres, form the blood–gas interface within the oxygenator. A trained perfusion technician controls the bypass machine.
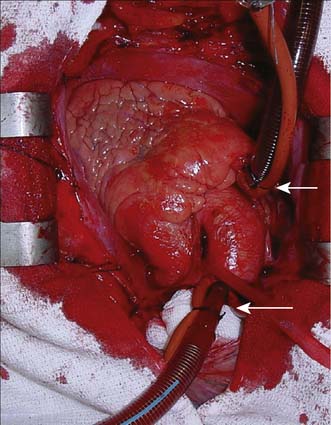
Fig. 22.3 Cannulation for cardiopulmonary bypass.
Upper arrow from right atrium to pump. Lower arrow from pump to aorta.
Postoperative care
Complications
Other than death or stroke, established complications include:
• bleeding – multifactorial causes including hypothermia, platelet dysfunction, CPB and pharmacological (aspirin, clopidogrel)
• low cardiac output – poor myocardial protection, previous poor left ventricular (LV) function
• arrhythmias – atrial fibrillation occurs in up to 40%
• infection – wound, respiratory
Recovery time
Patients undergoing routine elective coronary or valve surgery will usually leave acute hospital care within one week. Those requiring more extensive surgery or emergency procedures may take longer to recover. Most patients will have undergone a median sternotomy (Fig. 22.4). This wound heals quickly and, as the sternal edges are approximated securely by wire or heavy sutures, chest discomfort eases rapidly. Leg vein donor sites may take longer to heal, particularly around the knee. By 2 weeks the patient should be able to walk a few hundred metres, and by 3 months should have returned to full activity, including work.
Acquired cardiac disease
Surgical intervention may be required in the management of:
Ischaemic heart disease
Coronary artery disease (CAD)
Coronary artery atheroma (Ch. 21) results in narrowing of the vessels and most patients will present for surgery because of angina or previous myocardial infarction (MI).
Assessment
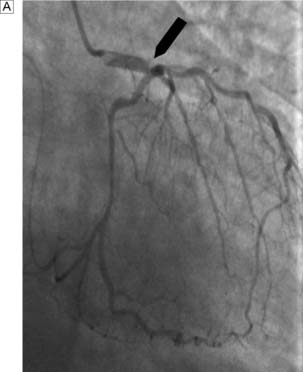
Exercise electrocardiography (ECG) is often used as an initial screening test for patients with suspected stable angina. Those with confirmed ischaemia then undergo coronary angiography and assessment of left ventricular function by means of angiography or echocardiography. Contrast medium is injected into the coronary circulation (Fig. 22.5) via a catheter that is usually inserted through the femoral or radial artery (Fig. 22.6). Images are obtained in several different planes so as to minimize the risk of missing eccentric lesions. Intervention is usually only advised for stenoses that exceed a 70% reduction in vessel diameter.
Coronary bypass
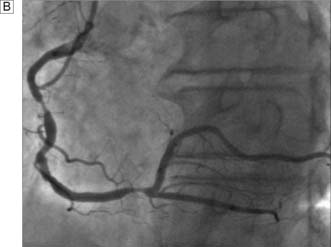
A coronary artery bypass graft (CABG) delivers blood to the distal coronary artery beyond a stenosis. If the distal artery is obliterated by atheroma, an endarterectomy procedure may be performed to restore the lumen. Originally, nearly all grafts comprised reversed segments of the long saphenous vein anastomosed proximally to the ascending aorta and distally to the coronary artery. Such grafts have patency rates of around 70% at 5 years and 40% at 10 years. Venous graft failure occurs as a result of intimal hyperplasia, which is thought to be, in part at least, a response to arterial pressure. The relatively high rate of vein graft failure stimulated interest in arterial grafts and led to the almost universal use of the internal thoracic artery (ITA). This is usually employed as a pedicled graft when it is left attached to the subclavian artery proximally, but can also be used as a free graft in the same manner as vein. ITA graft patency exceeds 90% at 5 years and 70% at 10 years. A common combination is to use the left ITA for the left anterior descending artery and vein grafts for the other vessels (Fig. 22.7).
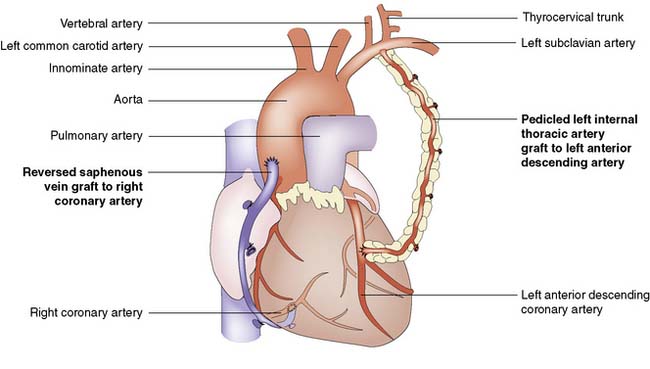
Fig. 22.7 Completed coronary bypass procedure with venous and left internal thoracic artery grafts in situ.
Results
Uncomplicated coronary surgery should carry a 2–3% risk of mortality and a 1–2% risk of stroke. Angina is relieved completely in about 70% of cases, is significantly improved in the remainder, and recurs with a frequency of about 10% per year. Successful revascularization may also improve breathlessness if it is related to myocardial ischaemia, and survival is probably enhanced in patients with left main stem and triple vessel disease. The use of arterial conduits is associated with better graft patency and improved survival. Although there is a trend in that direction for patients with multiple arterial grafts followed up beyond 10 years, the added benefit over one ITA graft placed to the left anterior descending coronary is small. This may reflect the progression of native coronary disease. Secondary prevention is mandatory in all patients with CAD and includes antiplatelet medication (aspirin) and cholesterol reduction (statin) (EBM 22.1).
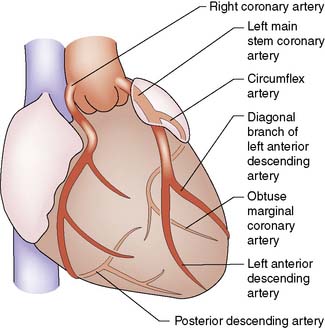
Summary Box 22.1 Coronary anatomy
• There are two coronary arteries (left and right), which have origin in the coronary sinuses: left or posterior sinus, right or anterior sinus
• The left main coronary artery passes behind the pulmonary trunk and divides into two large branches: the left anterior interventricular artery or left anterior descending (LAD), which supplies the anterior left ventricle and anterior two-thirds of the interventricular septum, and the circumflex coronary, which supplies the posterior and lateral walls of the left ventricle
• The right coronary artery passes down anteriorly in the right atrioventricular groove supplying the anterior right ventricle and acute marginal branches
• Either the right or circumflex may terminate as the posterior descending artery which supplies the inferior surface of both ventricles and the lower septum. This artery is then considered to be dominant
• The right, LAD and circumflex are each considered to be a ‘vessel system’. Disease within any one of these three vessels or its branches is termed single-vessel disease. Similarly, two- and three-vessel disease indicates involvement of two and three systems, respectively.
Surgery for the complications of coronary artery disease
Mitral valve regurgitation (MR)
Acute
Acute myocardial infarction involving a papillary muscle may cause this to rupture, causing gross regurgitation. The patient is usually very unwell with pulmonary oedema due to MR and low cardiac output due to infarction, and often requires emergency ventilation. Emergency mitral valve replacement and CABG is associated with a mortality of 15–40%, mainly due to poor ventricular function and secondary multiorgan failure (EBM 22.2).
22.2 Valve replacement
• Aortic valve replacement (AVR) is indicated for symptomatic patients with severe aortic stenosis (AS).
• AVR is indicated for patients with severe AS undergoing coronary artery bypass surgery (CABG) or surgery on the aorta or other heart valves.
• AVR is indicated for symptomatic patients with severe aortic regurgitation (AR) irrespective of left ventricular (LV) systolic function.
• AVR is indicated for asymptomatic patients with chronic severe AR and LV systolic dysfunction or while undergoing CABG or surgery on the aorta or other heart valves.
• Mitral valve (MV) surgery (repair if possible) is indicated in patients with symptomatic moderate or severe mitral stenosis.
• MV surgery is recommended for the symptomatic patient with acute severe mitral regurgitation (MR).
• MV surgery is beneficial for patients with chronic severe MR
Cardiac valvular disease
Surgical management
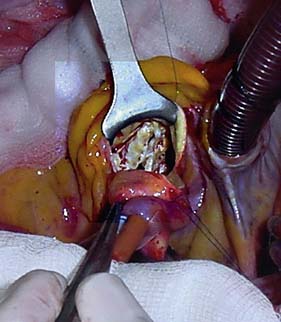
Options include valve replacement or repair. Replacement utilizes either a mechanical or a biological prosthesis. Mechanical valves have developed from the original ball-in-cage design through single disc designs to the current range of carbon bi-leaflet devices (Fig. 22.8). These should last indefinitely, but patients require lifelong warfarin to prevent thrombotic occlusion or embolism. Embolism risk is about 1–6% per year and is influenced by how accurately the INR is controlled. Mechanical valves produce audible clicks.
Biological valves are derived from:
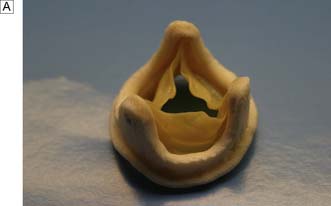
• glutaraldehyde-preserved porcine aortic valves mounted on a frame (stent) (Fig. 22.9A)
• glutaraldehyde-preserved bovine pericardium formed into a three-leaflet valve and mounted on a stent
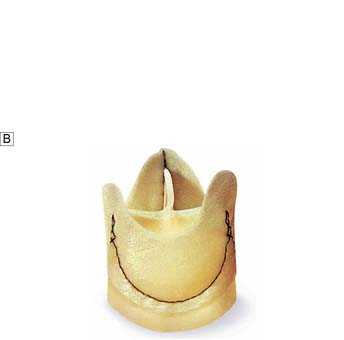
• glutaraldehyde-preserved porcine aortic unstented valves (Fig. 22.9B)
• human aortic root homografts removed from cadaveric hearts and preserved in antibiotic solution.
Aortic valve disease
Stenosis
Aortic stenosis is the commonest indication for valve surgery in the UK. Although rheumatic disease remains a common problem in underdeveloped countries, the most frequent aetiology in the Western world is calcific aortic stenosis which develops in the older population usually over 70 years. The normal aortic valve has three cusps but a congenital bicuspid valve usually calcifies from the sixth decade onwards (Fig. 22.10). Aortic stenosis causes left ventricular hypertrophy, effort angina, episodes of arrhythmia with syncope or even sudden death, and left ventricular failure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree