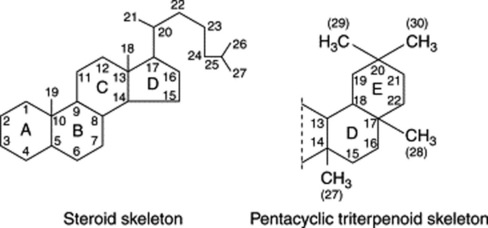
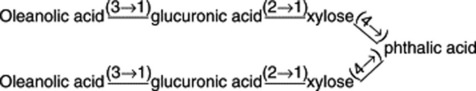Chapter 23 Saponins, cardioactive drugs and other steroids
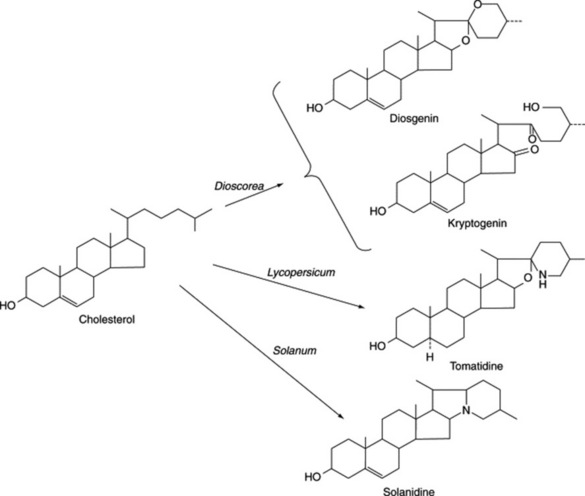
A distinct subgroup of the steroidal saponins is that of the steroidal alkaloids which characterize many members of the Solanaceae. They possess a heterocyclic nitrogen-containing ring, giving the compounds basic properties (as an example see solasodine, Fig. 23.5).
STEROIDAL SAPONINS
The steroidal saponins are less widely distributed in nature than the pentacyclic triterpenoid type. Phytochemical surveys have shown their presence in many monocotyledonous families, particularly the Dioscoreaceae (e.g. Dioscorea spp.), Agavaceae (e.g. Agave and Yucca spp.) and Smilacaceae (Smilax spp.). In the dicotyledons the occurrence of diosgenin in fenugreek (Leguminosae) and of steroidal alkaloids in Solanum (Solanaceae) is of potential importance. Some species of Strophanthus and Digitalis contain both steroidal saponins and cardiac glycosides (q.v.). Examples of saponins and their constituent sugars are given in Table 23.1.
Table 23.1 Examples of steroidal saponins.
| Steroidal saponin | Sugar components | Occurrence |
|---|---|---|
| Sarsaponin (Parillin) | 3 glucose, 1 rhamnose | Smilax spp. |
| Digitonin | 2 glucose, 2 galactose, 1 xylose | Seeds of Digitalis purpurea and D. lanata |
| Gitonin | 1 glucose, 2 galactose, 1 xylose | Seeds and leaves of D. purpurea and seeds of D. lanata |
| Dioscin | 1 glucose, 2 rhamnose | Dioscorea spp. |
As with cardiac glycosides, the stereochemistry of the molecule is of some importance, although not so much so for cortisone manufacture. Natural sapogenins differ only in their configuration at carbon atoms 3, 5 and 25, and in the spirostane series the orientation at C-22 need not be specified (cf. steroidal alkaloids). Mixtures of the C-25 epimers—for example, diosgenin (Δ5,25α-spirosten-3β-ol) and yamogenin (Δ5,25β- spirosten-3β-ol)—are of normal occurrence and their ratio, one to the other, is dependent upon factors such as morphological part and stage of development of the plant. In some instances in the plant, the side-chain which forms ring F of the sapogenin is kept open by glycoside formation as in the bisdesmosidic saponin sarsaparilloside of Smilax aristolochiaefolia.
BIOGENESIS OF STEROIDAL SAPONINS
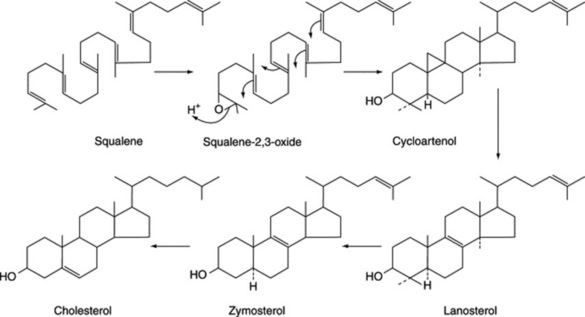
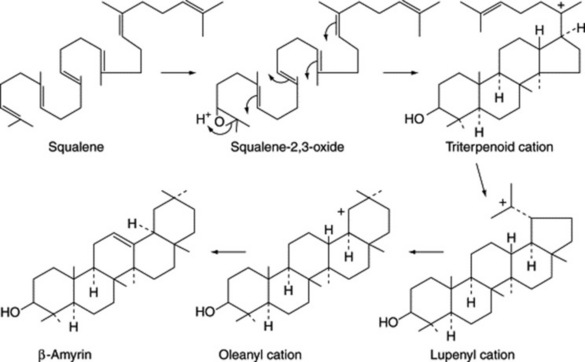
Steroidal saponins arise via the mevalonic acid pathway; the preliminary stages have been discussed in Chapter 18. A scheme for the subsequent cyclization of squalene to give cholesterol is illustrated in Fig. 23.1. Cholesterol, the wide distribution of which in plants has only relatively recently been shown, can be incorporated into a number of C27 sapogenins without side-chain cleavage (Fig. 23.2), although it is not necessarily an obligatory precursor. Extensive investigations involving whole plants, homogenates and cell cultures have been performed to elucidate these detailed pathways, including the origin of the 25-epimers (e.g. diosgenin and yamogenin).
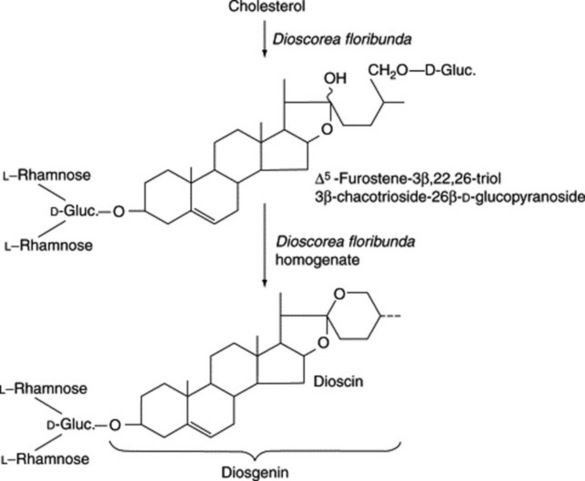
As early as 1947 Marker and Lopez had postulated that steroidal saponins exist in plants in a form where the side-chain is held open by glycoside formation. However, direct evidence for the natural occurrence of these compounds was not forthcoming for another 20 years. It has been shown that such open-chain saponins are, like the more common ones, formed from cholesterol. In Dioscorea homogenates one such compound has been converted to dioscin (a diosgenin glycoside) (Fig. 23.3).
NATURAL STEROIDS FOR THE PRODUCTION OF PHARMACEUTICALS
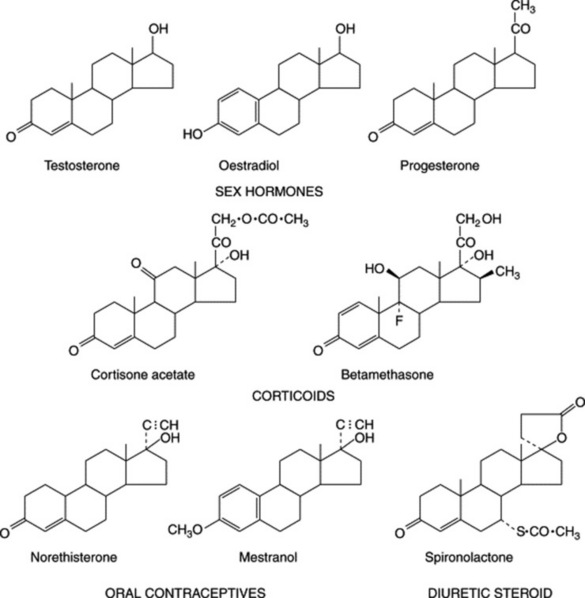
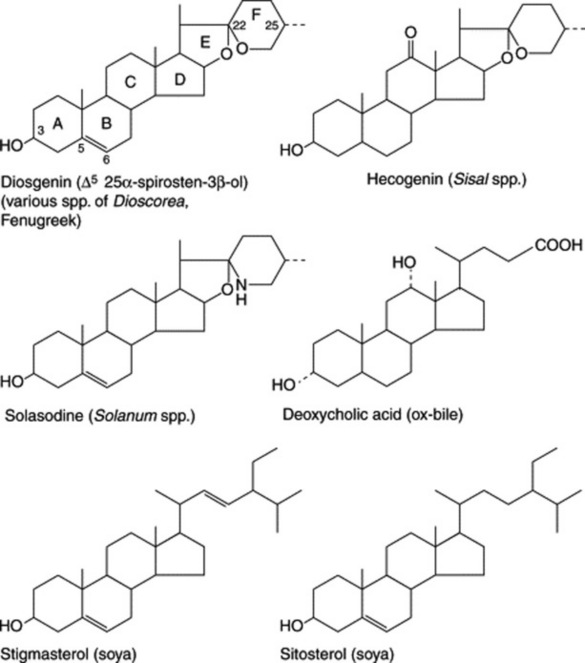
As indicated in Fig. 23.4, which illustrates the range of steroids required medicinally, cortisone and its derivatives are 11-oxosteroids, whereas the sex hormones, including the oral contraceptives, and the diuretic steroids have no oxygen substitution in the C-ring. Fig. 23.5 shows some of the more important natural derivatives which are available in sufficient quantity for synthetic purposes. Hecogenin with C-ring substitution provides a practical starting material for the synthesis of the corticosteroids, whereas diosgenin is suitable for the manufacture of oral contraceptives and the sex hormones. Diosgenin, however, can also be used for corticosteroid synthesis by the employment, at a suitable stage in the synthesis, of a microbiological fermentation to introduce oxygen into the 11α-position of the pregnene nucleus.
Efforts are constantly being made to discover new high-yielding strains of plants and to assure a regular supply of raw material by the cultivation of good-quality plants. Hardman in a review on steroids (Planta Med., 1987, 53, 233) recorded that, annually, the American Chemical Abstracts contained some 3000 references pertinent to plant steroids or related compounds. Some of the better-known examples of steroidal sapogenins and their sources are given in Table 23.2. (For a review, tabulating over 200 sapogenins, see A. V. Patel et al., Fitoterapia, 1987, 58, 67.)
Table 23.2 Some steroidal sapogenins and their sources.
| Sapogenin | Species | Location |
|---|---|---|
| Diosgenin | Dioscorea sylvatica | Transvaal and Natal |
| D. mexicana and D. composita | Mexico and Central America | |
| D. collettii, D. pathaica and D. nipponica | China | |
| D. floribunda | Guatemala and cultivated in India | |
| D. deltoidea and D. prazeri | India | |
| D. tokoro | Japan | |
| Costus speciosus | India | |
| Kallstroemia pubescens | Tropical America; introduced into West Brazil | |
| Trillium spp. | North America | |
| Trigonella foenum-graecum | India, Egypt, Morocco | |
| Hecogenin | Agave sisalana | Subtropical America and cultivated in Kenya for sisal and saponin |
| A. rigida | Mexico | |
| Hechtia texensis | Central America | |
| Sarsapogenin | Yucca spp., Smilax spp. | Central America |
| Sarmentogenin | Strophanthus spp. | Africa |
Dioscorea species
Tubers of many of the dioscoreas (yams) have long been used for food, as they are rich in starch. In addition to starch, some species contain steroidal saponins, others alkaloids. From a suitable source the sapogenins are isolated by acid hydrolysis of the saponin. Previous fermentation of the material for some 4–10 days often gives a better yield. The water-insoluble sapogenin is then extracted with a suitable organic solvent. Both wild and cultivated plants are used. Cultivation requires attention to correct soil and drainage, support for the vines and freedom from weeds, virus, fungus and insect attack. According to the species, the tubers reach maturity in 3–5 years and on average, yield 1–8% of total sapogenin.
Until 1970 diosgenin isolated from the Mexican yam was the sole source for steroidal contraceptive manufacture. With the nationalization of the Mexican industry, however, prices were increased to such an extent that manufacturers switched to hecogenin for corticosteroids, to other sources of diosgenin and to the use of the steroidal alkaloids of Solanum species. Total synthesis also became economically feasible and is now much used. More recently, the economics of steroid production have again changed in that China is now exporting large quantities of diosgenin; it is of high quality, being free of the 25β-isomer yamogenin, although this is of no commercial significance, and is reasonably priced. Three of the many Dioscorea spp. found in China and used commercially are given in Table 23.2; the tubers of these yield 2% of diosgenin, with the average content of diosgenin for the main areas of production (Yunnan Province and south of the Yangtze River) being 1%.
FENUGREEK
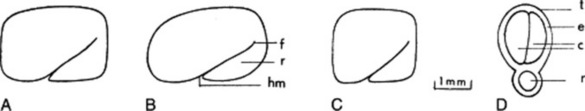
The very hard seeds have a strong characteristic odour and are irregularly rhomboidal and oblong or square in outline. They are somewhat flattened and are divided into two unequal parts by a groove in the widest surfaces. Their shape and size, and position of the embryo and hilum are shown in Fig. 23.6. The BP/EP describes the seeds as brown to reddish-brown but in commerce olive-green or yellow-brown samples are oftenencountered. Microscopically the testa and hypodermis are characteristic and the mucilage-containing endosperm enables the swelling index (q.v.) of the seeds (not less than six) to be used as a test of purity.
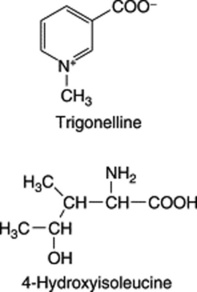
Fenugreek contains the simple pyridine-type alkaloid trigonelline; this base, also reported in garden peas, hemp seed, coffee and many other products was first isolated and described by Jahns in 1885. Today it serves as the reference substance for the BP/EP TLC identification test for the drug. Pharmaceutical manufacturing interest lies in a number of steroidal sapogenins, particularly diosgenin which is contained in the oily embryo. Fazli and Hardman investigated a number of commercial samples of seed as possible commercial sources of diosgenin (see reference in Fig. 23.6) and reported contents of 0.8–2.2% expressed on a moisture-free basis. In 1986, Gupta and colleagues isolated a series of furostanol glycosides (F-ring opened) named trigofoenocides A–G. In a series of papers, further furostanol glycosides designated trigoneosides Ia, Ib–Xlla have been reported in Egyptian seeds (T. Murakami et al., Chem. Pharm. Bull., 2000, 48, 994). As with dioscoreas, the yield of diosgenin is increased by fermentation of the seeds prior to acid hydrolysis. Although the diosgenin yield is lower than that of the dioscoreas, fenugreek is an annual plant which will also give fixed oil, mucilage, flavouring extracts and high-protein fodder as side-products. A number of Hardman’s registered varieties have been subjected to field trials in the UK. However, as long as other cheap sources of diosgenin are available commercially, fenugreek must be regarded as a fall-back source for this sapogenin.
Solanum species
This large genus (over 1000 spp.) is noted for the production of C27 steroidal alkaloids in many species. Some of these alkaloids are the nitrogen analogues of the C27 sapogenins (e.g. solasodine and diosgenin: Fig. 23.5). Another series of C27 compounds contain a tertiary nitrogen in a condensed ring system (e.g. solanidine; Fig. 23.2). These compounds can also be employed in the partial synthesis of steroidal drugs, and a number of companies have devoted considerable attention to commercial production. Species so exploited are Solanum laciniatum, S. khasianum (a nearly spineless variety has been produced) and S. aviculare; trials on the production of S. marginatum have been conducted in South America. Zenk and colleagues have assayed over 250 spp. of Solanum for solasodine. A number of new glycosides have been isolated from S. dulcamara leaves and include two based on tigogenin and two on soladulcidine.
Soya bean sterols
The soya (soy, soja) plant, Glycine max (G. soya) (Leguminosae) is extensively cultivated for its seeds, which are rich in oil and protein. The seeds also contain appreciable quantities of the phytosterols stigmasterol and sitosterol (Fig. 23.5). Although not sapogenins, they are included here because they are now used extensively for steroid synthesis. They are obtained as byproducts of soap-making, being components of the unsaponifiable matter of the fixed oil. Pure stigmasterol, with its unsaturated side-chain is amenable to chemical conversion to suitable starting materials and can replace diosgenin. But it was more recently that sitosterol, the saturated side-chain of which could not be removed chemically without ring fragmentation, became commercially useful as the result of the discovery of a suitable microbiological side-chain removal. Both phytosterols are now processed by microorganisms. Similar phytosterols are found in other products—for example, cotton-seed oil, tall-oil (from the wood-pulp industry) and sugarcane wax.
For details of the soya isoflavones and their dietary importance, see Chapter 32: The plant nutraceuticals.
Sarsaparilla root
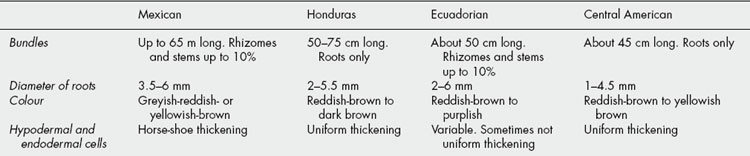
Sarsaparilla consists of the dried roots and sometimes also of the rhizomes of species of Smilax (Liliaceae, modern authors, Smilacaceae). The determination of the exact geographic and botanical sources of the numerous varieties which have from time to time been imported has been a matter of some difficulty (see Table 23.3).
Table 23.3 Varieties of sarsaparilla.
| Variety and geographic source | Synonyms | Botanical source |
|---|---|---|
| Mexican (Southern Mexico, Guatemala, British Honduras) | Vera Cruz or Grey | Smilax aristolochiaefolia |
| Honduras (Guatemala, British Honduras, Honduras, cultivated in Jamaica) | Brown | S. regelii |
| Ecuadorian and Peruvian | Guayaquil | S. febrifuga |
| Central American | Costa Rica or ‘Jamaican’ | Undetermined spp. |
The plants produce numerous roots, 3 m or so long, which are attached to a short rhizome. The roots are cut, sufficient, however, remaining in the ground for the plant to resume its growth. Sometimes the rhizomes as well as the roots are collected. After drying in the sun the drug is made into bundles and the bundles into bales.
Sarsaparilla is imported in large bales bound with wire. Each bale usually contains numerous bundles of approximately uniform size. These consist of long roots, with or without pieces of rhizome and aerial stems. The commercial varieties (Table 23.4) differ from one another in colour, ridges and furrows; in the presence or absence of rhizome and aerial stems; in the relative proportions of cortex, wood and pith, as seen in transverse section; in their microscopical structure. The drug is nearly odourless but has a somewhat sweetish and acrid taste. Owing to the presence of saponins, aqueous extractives froth readily.
BUTCHER’S BROOM
Microscopical characteristics, as seen in the powder, include cells with thickened beaded walls with large oval pits, thin-walledparenchyma containing calcium oxalate, thick-walled fibres, and small vessels.
ELEUTHEROCOCCUS
Constituents
The rhizomes and roots contain a number of constituents termed eleutherocides (A to G; M). These, however, are not all of the same chemical group but include the phenyl propane glycosides eleutheroside B (syringin) and its aglycone sinapyl alcohol; also the lignans (−)-syringaresinol diglucoside (E) and its stereoisomer (D) (formulae Table 21.7), together with caffeic acid and its ethyl ester, chlorogenicacid and coniferaldehyde. Coumarins include coumarin, isofraxidin 7-glucoside (B1) and sesamin (B4). A group of heteroglycans (eleutherans A to G) have been studied for their hypoglycaemic activity. Other constituents include hedera-saponin (M), daucosterol (A) and volatile oil, about 0.8%.
GINSENG
Constituents
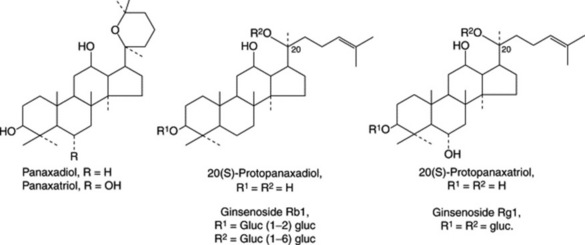
P. ginseng roots have been thoroughly studied by modern methods of analysis and, of the many compounds isolated, the medicinal activity appears to reside largely in a number of dammarane-type saponins termed ginsenosides by Japanese workers and panaxosides by Russian workers. These two series of compounds, all now generally termed ginsenosides, are glycosides respectively derived from the diol 20(S)-protopanaxadiol and the triol 20(S)-protopanaxatriol. Examples of the former are ginsenosides Rb1, Rb2 and Rb4 and of the latter ginsenosides (panaoxosides) Re, Rf, Rg1, Rg2 (see Fig. 23.7). Acid hydrolysis of these saponins involves ring closure of the aglycone giving either panaxadiol or panaxatriol (Fig. 23.7). Some 30 ginsenosides have been named, although not all fit into the above scheme, e.g. ginsenoside Ro is an oleanolic acid derivative. Glucose is the principal sugar involved with some input of arabinose and rhamnose.
Two other groups of compounds present in the root which have known therapeutic activity are high molecular weight polysaccharides (glycans) and acetylenic compounds. The glycans of P. ginseng have been named panaxans (A–U); panaxans A and B have been shown to be constituted mainly of α-(1 → 6) linked D-glucopyranose units with C-3 branching and a small component of peptide. (For the isolation of other polysaccharides termed ginsenans see M. Tomoda et al., Biol. Pharm. Bull., 1993, 16, 1087.) Those glycans tested have hypoglycaemic, antiulcer and immunological properties. One acidic polysaccharide MW 150000, originally isolated in 1993, and composed of 3.7% protein, 47.1% hexoses and 43.1% galacturonic acid, has antineoplastic immuno-stimulant properties.
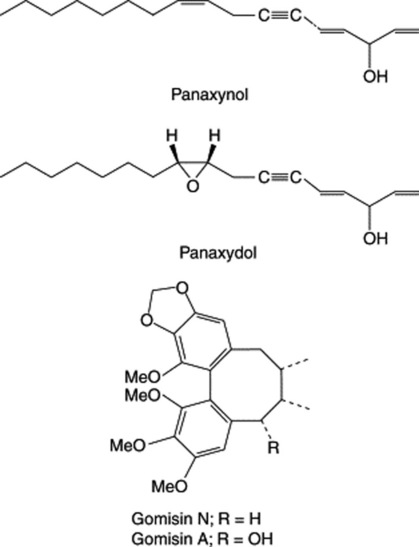
A considerable number of mainly C17, but also C14, polyacetylenic alcohols have been isolated from the roots in recent years and are typified by panaxynol and panaxydol (Fig. 23.8). These compounds have been shown to have antitumour properties and Japanese patents exist for their isolation and derivatization. The cytotoxic activity of the C17-polyacetylenes against leukaemia cells has been shown to be almost 20 times greater than that for the C14-compounds (Y. Fujimoto et al., Phytochemistry, 1992, 31, 3499). K. Hirakura et al. have characterized a series of polyacetylenes named ginsenoynes A–K (see Phytochemistry, 1992, 31, 899 and references cited therein) and subsequently (ibid., 1994, 35, 963) three new linoleoylated polyacetylenes.
Other constituents include sesquiterpenes (panacene, β-elemene, panasinsanol A and B, ginsenol, etc.) to be found in the volatile oil (0.05–0.1%), together with various monoterpenes and monoterpene alcohols. Three minor sesquiterpenes recently identified are panaxene, panaginsene and ginsinsene (R. Richter et al., Phytochemistry, 2005, 66, 2706). Lignans of the dibenzocyclooctadiene type have been isolated from Korean Red Ginseng (Fig. 23.8). Minor components isolated from ginseng roots include sterols, vitamins of the D group, flavonoids and amino acids.
Allied species
Panax quinquefolium root is one of the major drugs of US foreign trade. It is produced in the eastern USA and Canada, 90% of the US cultivated drug coming from north-central Wisconsin. Some of the ginsenosides of this species are the same as those of the Chinese and Korean drug; others appear to differ. In addition to 14 known dammarane-type saponins, M. Yoshikawa et al. (Chem. Pharm. Bull., 1998, 46, 647) identified a further five new compounds designated quinquenosides I–V. D. Don et al. (Chem. Pharm. Bull., 2006, 54, 751) have reported on a new dammarane-type saponin, ginsenoside Rg8, together with other known ginsenosides. In 1997, Kitanaka et al. showed the hybrid P. ginseng × P. quinquefolium to be superiorto either parent in production of ginsenosides. However, as the plant is sterile, root cultures were established and these showed a comparable ginsenoside production to the field grown material (D. Washida et al., Phytochemistry, 1998, 49, 2331).
Panax japonicum and P. japonicum var. major contain chikusetsusaponins, ginsenosides and glycans.
PENTACYCLIC TRITERPENOID SAPONINS
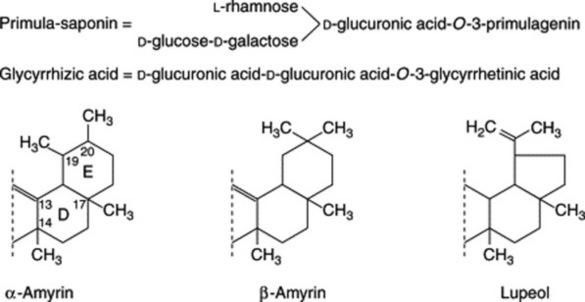
In these saponins the sapogenin is attached to a chain of sugar or uronic acid units, or both, often in the 3-position, as in the examples above. Biosynthesis, as with the steroids, involves ring-closure of squalene and is illustrated in Fig. 23.9.
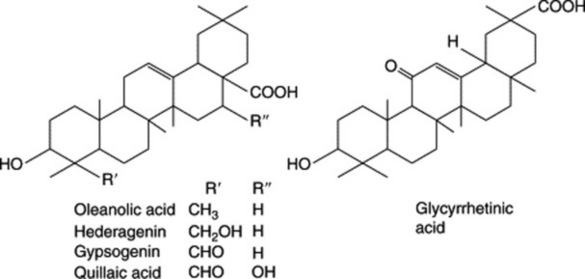
The related triterpenoid acids are formed from these by replacement of a methyl group by a carboxyl group in positions 4, 17 or 20 (Fig. 23.10).
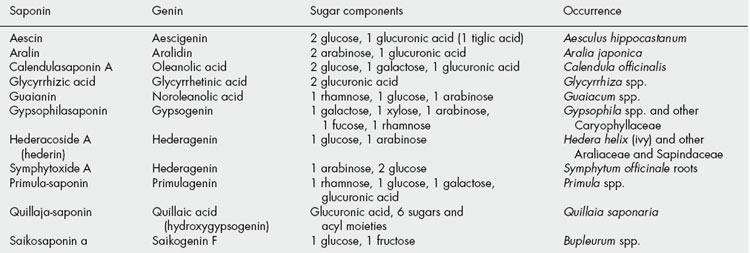
Plant materials often contain these saponins in considerable amounts. Thus, primula root contains about 5–10%; liquorice root about 2–12% of glycyrrhizic acid (and a correspondingly larger amount of glycyrrhizin, the potassium calcium salt); quillaia bark up to about 10% of the mixture known as ‘commercial saponin’; the seeds of the horse-chestnut up to 13% of aescin. As some plants contain more than one saponin and purification is often difficult, the structures of even some of the well-known saponins given in Table 23.5 have only recently been established. Oleanolic acid also occurs as a saponin in sugar beet, thyme, Guaiacum spp. (also in the nor-form), and in the free state in olive leaves and clove buds.
LIQUORICE ROOT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree