Patricia C. Seifert
Cardiac Surgery
Cardiac surgery is increasingly moving toward interventional procedures that use minimally invasive approaches and percutaneous access routes. The introduction of coronary angioplasty in the 1970s accelerated interventional technology and stimulated perioperative clinicians to expand both traditional and innovative treatment options. These changes have also encouraged individuals to refine anastomotic techniques, investigate new routes to achieve cardiopulmonary bypass and vascular repair, design more tissue-friendly instrumentation, and create operative arenas that embrace aspects of both the typical operating room (OR) and the newer interventional suites. These newer, “hybrid” ORs have stimulated cardiac clinicians to expand their skill sets, develop closer working relationships with colleagues in the cardiac catheterization and electrophysiology laboratories, and reassess how these changing technologies can strengthen the delivery of patient-focused care (Weisse, 2011). Other advances have included the growth of precise, three-dimensional diagnostic images; improved monitoring of neurologic and renal function; effective blood conservation strategies; refined anesthetic and cardiac drugs; and an overall greater understanding of the causes and treatment of glucose abnormalities, antibiotic-resistant infections, and other abnormalities that affect patient outcomes (Hatton et al, 2011; Nalla et al, 2012; Nussmeier et al, 2009; Teirstein and Lytle, 2012).
Other trends that have significantly affected cardiac surgery include heightened emphases on safety, education, quality outcomes, staff competence, and cost-effectiveness (Speir et al, 2009). The use of a simple checklist to ensure correct identification of the patient or that performance of a central line insertion is in a manner that eliminates or minimizes the risk of a catheter-based infection (Lipitz-Snyderman, 2011) are among the many safety initiatives that produce better patient outcomes.
This chapter describes both traditional and newer, innovative treatment options for coronary artery disease (CAD), valvular dysfunction, thoracic aneurysms, conduction disturbances, heart failure, and end-stage cardiac disease. Therapeutic interventions may use laser, radiofrequency, or cryothermal energies. Stem cell therapy, used to induce cardiac neorevascularization at the cellular level (Jeevanantham et al, 2012), and “destination” mechanical assist devices (which remain implanted)—or devices that can result in the “recovery” of the ventricle—are among the newer areas of investigation showing great promise (Acker and Jessup, 2012).
Surgical Anatomy
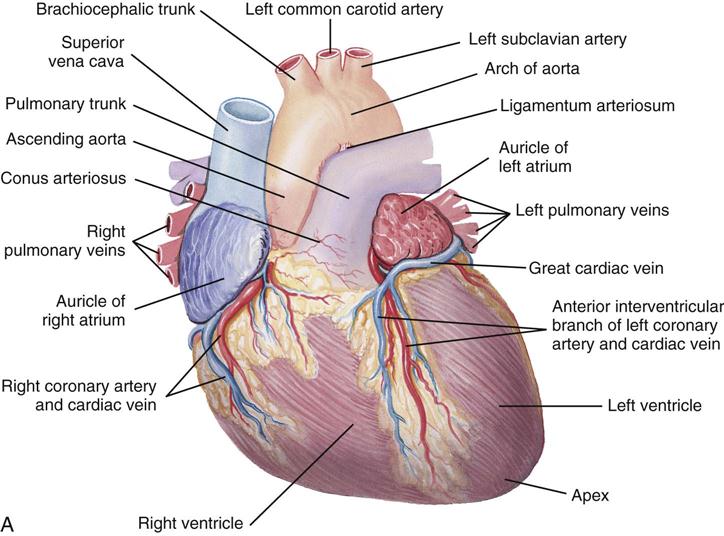
The heart (Figure 25-1) is a four-chamber muscular pump that propels blood into the systemic and pulmonary circulatory systems. It sits within a pericardial sac within the mediastinum, which lies between the lungs, posterior to the sternum, and anterior to the vertebrae, esophagus, and descending portion of the aorta. The location of the diaphragm is below the heart (Figure 25-2). The heart bears attachments at the aorta, pulmonary artery, superior and inferior venae cavae, and the pulmonary veins; the ventricles are relatively mobile, enabling the surgeon to rotate the walls of the ventricles and the apex of the left ventricle in order to assess (and graft) coronary arteries to the lateral and posterior aspects of the myocardium.
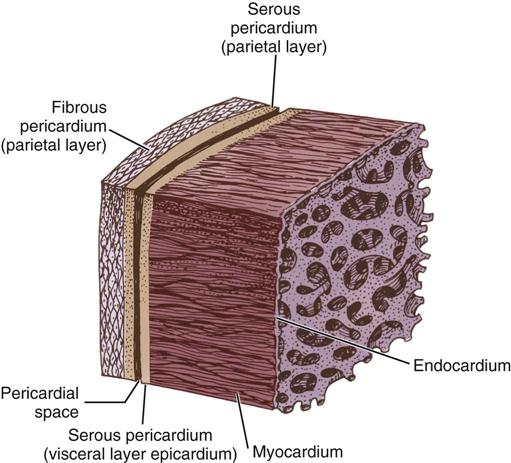
The cardiac wall consists of three layers: the epicardium or outer lining; the myocardium or muscular layer, an important functional layer; and the endocardium or inner lining (Figure 25-3). The left ventricular muscle layer has the most depth (i.e., thickness) of the four chambers and can generate the greatest pressure, required to pump blood into the circulatory system.
Two thirds of the heart are to the left of the midline, and the remaining one third is to the right. Although functionally divided into right and left halves, the heart sits rotated to the left, with the right side anterior and the left side relatively posterior. Injury to the anterior chest (e.g., from blunt force or stab wounds) is likely to damage the right ventricle. This may prove significant for myocardial repair because the right ventricle has lower pressures than the left, thus allowing additional time for repair before exsanguination.
Each half of the heart contains an upper and a lower communicating chamber—the atrium and ventricle, respectively. The right atrium (RA) receives desaturated blood from the inferior and superior venae cavae and from coronary circulation via the coronary sinus. The left atrium (LA) receives oxygenated blood from the lungs by way of the pulmonary veins. From both atria, blood then flows through the atrioventricular (AV) valves into the ventricles.
The left ventricle (LV) pumps oxygenated blood into the major vessels of the systemic circulatory system by way of the aorta and its main branches to the head, upper extremities, abdominal organs, and lower extremities. The right and left internal mammary arteries (IMAs) (thoracic), used as grafts during coronary bypass surgery, branch off the subclavian arteries and course behind and parallel to the edges of the sternum. The arteries of the circulatory system subdivide into arterioles and eventually into capillaries and the individual cells, where internal respiration and metabolic exchange occur. From the cells and capillary beds, desaturated blood flows into the venules and veins and finally returns to the RA.
In the pulmonary circulatory system, blood pumps from the right ventricle (RV) through the pulmonary valve into the main pulmonary artery (PA). The PA divides into the right and left pulmonary arteries, which further subdivide into the arterioles and capillaries of the lungs. External respiration occurs in the capillary beds and the alveoli, wherein carbon dioxide exchanges for oxygen. Freshly oxygenated blood from the lungs then flows through the pulmonary veins into the LA.
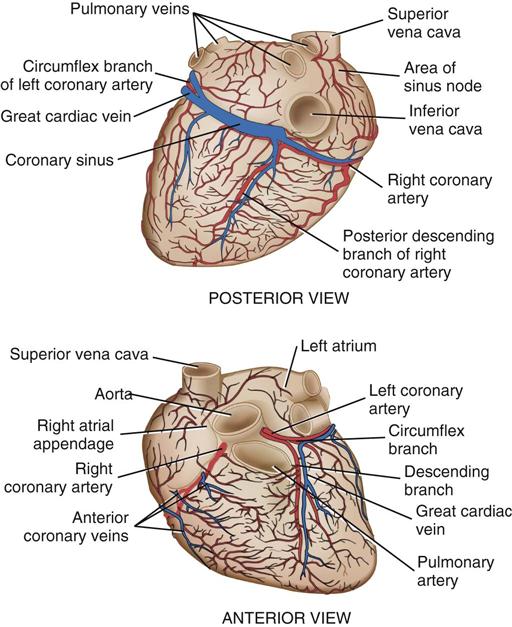
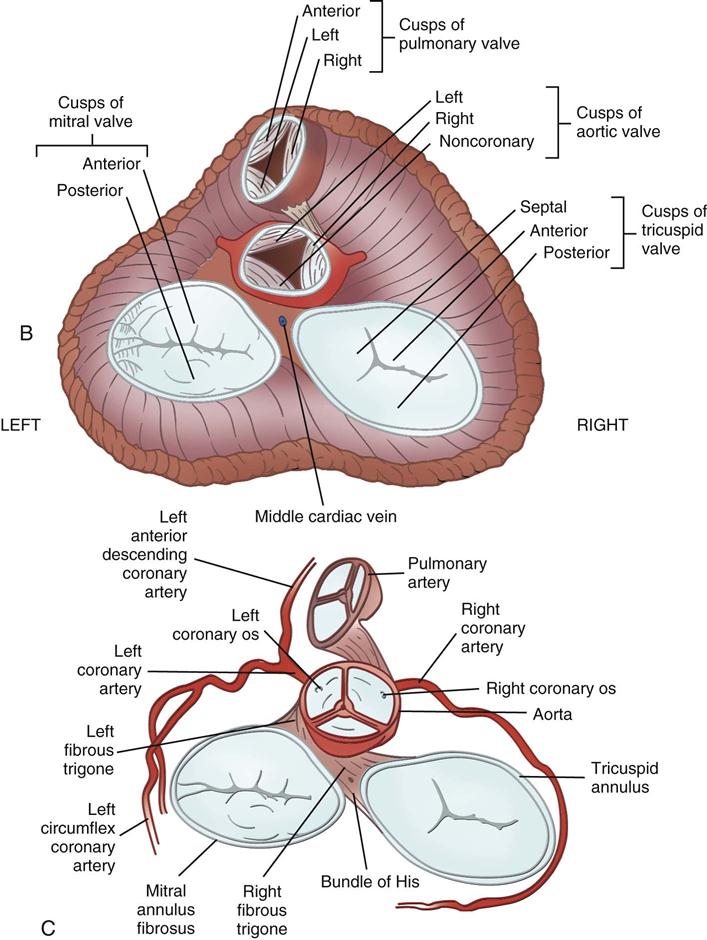
Coronary circulation (Figure 25-4) supplies oxygen and nutrients to, and removes metabolic waste from, the myocardium; internal respiration occurs in the myocytes. The heart receives its blood supply from the left and right coronary arteries, which originate in the corresponding left and right sinuses of Valsalva behind the cusps of the aortic valve in the ascending aorta. The left main coronary artery divides into the left anterior descending (LAD) coronary artery and the circumflex coronary artery; with the right coronary artery (RCA), these arteries are the three main vessels of the coronary arterial system.
In coronary arteries affected by CAD, focal or diffuse atherosclerotic plaques develop and progressively enlarge, diminishing myocardial blood flow and oxygenation, which produces ischemic pain in many, but not all, cases. Occlusion of a coronary artery by expanding atherosclerotic lesions causes myocardial infarction (MI) and irreversible damage to the region of the myocardium perfused by the obstructed artery. Coronary artery bypass procedures increase blood flow to the affected ischemic areas by attaching a bypass graft conduit to the artery distal to the narrowed portion of the artery. A totally occluded (infarcted) artery does not benefit from bypass surgery because the myocardial injury is irreversible if not treated (e.g., via revascularization) within 6 hours (Jneid et al, 2012). The main coronary arteries sit in the epicardium, which facilitates accessibility during coronary bypass procedures. From these arteries arise the septal perforators and other branches that penetrate the entire myocardium. The cardiac veins empty into the RA by way of the coronary sinus; the thebesian veins, prominent in the walls of the RA and the RV, open directly into these chambers.
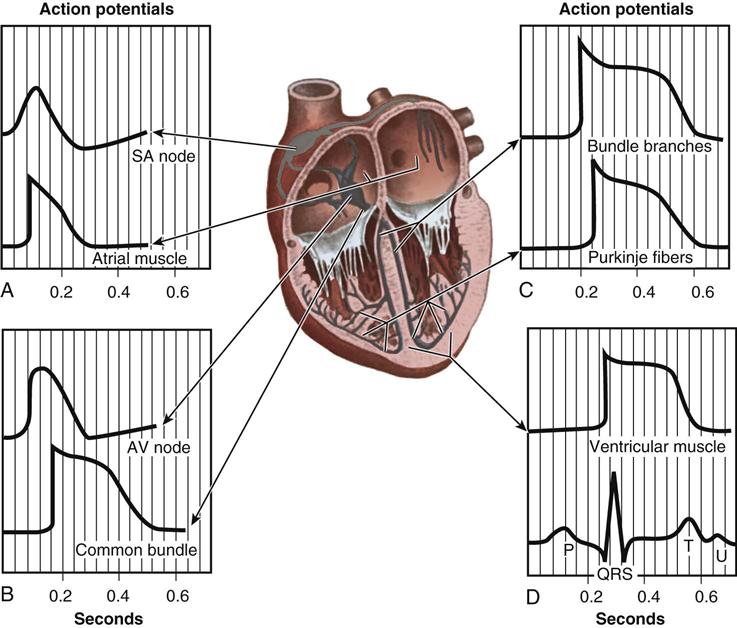
From the medulla oblongata, nerve impulses to the heart travel along the middle cervical nerve, composed of sympathetic fibers, and the vagus nerve, composed of parasympathetic fibers. Sympathetic fibers increase the force and rate of contraction, whereas parasympathetic fibers control heart rate. Running vertically along the right and left sides of the pericardium are major branches of the phrenic nerve, which innervate the diaphragm and stimulate it to contract. In order to protect the diaphragm, identifying the phrenic nerve is important in procedures that involve incision or excision of the lateral pericardium. Within the myocardium itself, certain areas of tissue undergo natural modification to form a conduction system (Figure 25-5). The process of excitation and contraction originates in the sinoatrial (SA) node, located in the area where the superior vena cava (SVC) meets the RA. The impulse spreads to the atria through internodal pathways and travels to the AV junction (which contains the AV node) located medial to the entrance of the coronary sinus in the RA, close to the tricuspid valve. From the AV junction, the impulse spreads to the bundle of His, which extends down the right side of the interventricular septum. The bundle of His divides into the right and left bundle branches, which terminate in a network of fibers called the Purkinje system. Purkinje fibers are spread throughout the inner surface of both ventricles and the papillary muscles, which when stimulated produce contraction of the heart muscle. Thus the location of conduction tissue is clinically significant during surgical repair of atrial or ventricular septal defects.
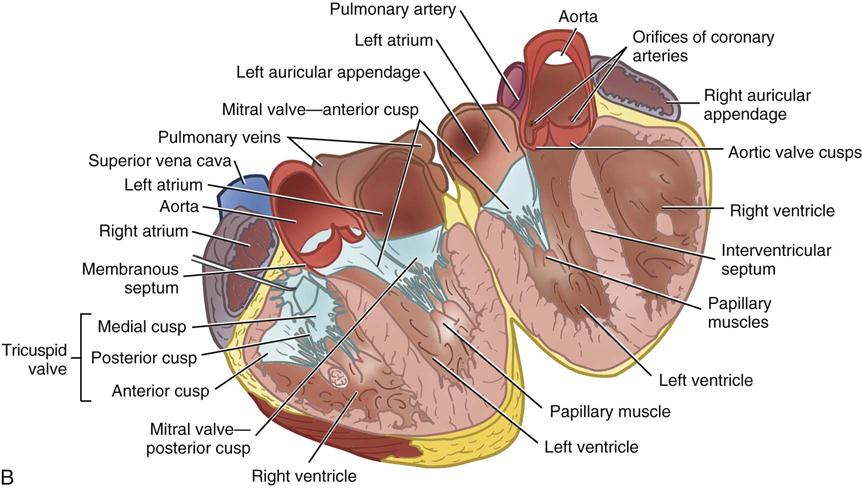
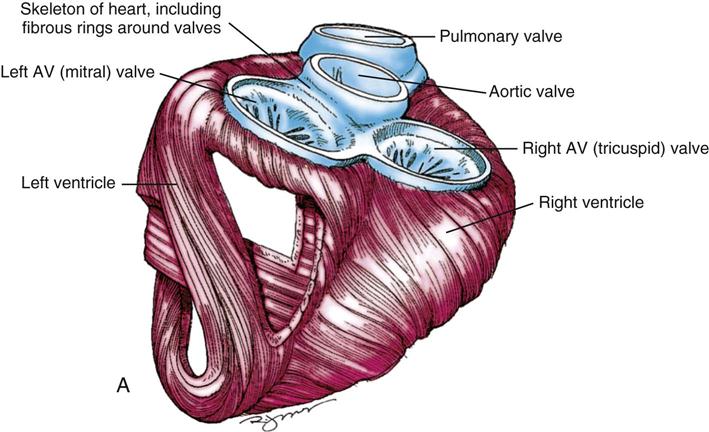
During myocardial contraction and relaxation, spiral fibers of the heart contract and relax (Figure 25-6, A). To prevent regurgitation of blood, the four cardiac valves (see Figure 25-6, B and C, and Figure 25-7) open and close to maintain unidirectional blood flow. The AV valves are between the atria and the ventricles. The right AV valve is called the tricuspid valve and contains three leaflets (or cusps). The left AV valve, called the mitral valve, consists of two leaflets (see Figure 25-6). Each AV valve is a complex system consisting of a fibrous annulus surrounding the valve orifice, the valve leaflets, the chordae tendineae, the atrium, and the papillary muscles, which anchor the valve to the inner ventricular wall (see Figure 25-1). The mitral valve annulus is a dynamic structure with a three-dimensional “saddle” shape, which has stimulated the design of newer prosthetic annuloplasty rings (Silbiger, 2012). When the ventricle contracts, these muscles and the chordae tendineae, connected to the valve leaflets, prevent the leaflets from everting into the atrium. All parts of the system must function for the valve to work properly. If the shape of the ventricle has been changed by dilation or hypertrophy, for example, the altered geometry of the ventricle impairs ventricular function. Conditions such as hypertension, myocardial injury, and aortic stenosis promote a pathologic remodeling of the heart that can lead not only to valvular dysfunction but also heart failure and malignant dysrhythmias (Gopaldas, 2012).
The semilunar valves are at the outlets of the LV and RV. These valves are known as the aortic and pulmonic valves, respectively. They have fewer components than the AV valves, and they open and close passively with cyclic fluctuations in blood pressure and volume that occur during systole and diastole.
Abnormalities such as stenosis, insufficiency, or a combination of both impair the mechanical function of the valves. Stenosed valves have leaflets that are fibrous and stiff, with uneven and adherent margins. Regurgitant, insufficient, or incompetent valves, such as those with leaflet degeneration or perforations, dilated annuli, or ruptured chordae tendineae, produce regurgitation of blood into the originating chamber. These conditions, or a combination of stenosis and insufficiency, strain the myocardium by increasing intracardiac pressure, volume, and workload. The sound of blood flowing through a narrowed or incompetent valve produces an abnormal sound called a murmur.
Any of the four valves may be deformed congenitally. Acquired valvular heart disease most commonly affects the mitral and aortic valves and appears to worsen with increased stress associated with the higher pressures within the left chambers of the heart. Although rare, primary tumors of the heart may impair valve function by partially occluding a valve orifice, causing incomplete closure of the leaflets. Portions of the tumor may break off and embolize. Most tumors are benign; the most common is the cardiac myxoma. Diagnostic imaging can identify many of these lesions, but the lesion may not cause symptoms to motivate a person to seek medical treatment (McManus, 2012).
Perioperative Nursing Considerations
Specialized nursing considerations that apply to thoracic surgery (see Chapter 23) and to vascular surgery (see Chapter 24) often also apply to cardiac surgery. Pediatric cardiac procedures appear in Chapter 26.
Assessment
As the severity of pathologic changes varies among patients throughout the life span, knowledge of physical status, psychosocial concerns, and functional health patterns enables the perioperative nurse to plan and manage patient care. The perioperative nursing data set should include the patient’s biopsychosocial history, the physical examination, and results from laboratory and diagnostic imaging tests.
History.
The history includes information about the patient’s health status as well as the response to the disease and the recommended intervention or interventions. Patients with cardiac disease may display such symptoms as ischemic chest pain (angina pectoris), fatigue, dyspnea, and syncope. Depending on their severity, these subjective symptoms affect the patient’s functional status and ability to engage in activities of daily living; the New York Heart Association’s Functional Classification System (Box 25-1) is often used to assess functional ability. Updates to the classification system also include objective measurements such as x-rays and echocardiograms (American Heart Association [AHA], 2012 a,b).
Atypical ischemic chest pain is more likely in women than in men, and angina may arise from vasospastic angina, mitral valve prolapse, or psychologic factors. CAD is unusual in premenopausal women; after menses ends, however, the risk is similar to that of men (AHA, 2013; Caboral, 2013; Mosca et al, 2011; Roger et al, 2012; Wenger, 2012). Controversy exists regarding menopausal hormone therapy. Estrogen hormone replacement therapy is not recommended in postmenopausal women, according to the Evidence-Based Guidelines for Cardiovascular Disease Prevention in Women: 2011 Update (Mosca et al, 2011) (Evidence for Practice). Greater emphasis is being placed on effectiveness of interventions as well as on the evidence supporting those interventions; effective control of blood pressure, diabetes management, and maintenance of optimal lipid levels are significant risk reduction interventions for women (Caboral, 2013).
Women undergoing coronary artery bypass graft (CABG) also have some unique challenges and experiences. According to Banner and colleagues (2011), some women may minimize any public display of illness in an attempt to preserve the appearance of normalcy; they even may hesitate to tell significant others about impending surgery until the day before admission. Postoperatively they may wish to return home early in order to resume regular activities. Once home, some women were unprepared for the limitations that they faced, both physical and social; the authors recommend focused advice, support, and education (Banner et al, 2011).
A cardiovascular disease risk factor profile (Box 25-2) is helpful to plan care for hospitalization and discharge by focusing on areas that might require further patient education. For example, diabetes is a risk factor because it affects the vascular system, may retard healing, and may predispose the patient to infection. Of special concern is the epidemic growth of type 2 diabetes in particular and the role of hyperglycemia in general. Although type 2 diabetes formerly was considered an adult-onset disease, children are increasingly vulnerable because of a greater incidence of increased body weight, sedentary lifestyle, and accelerated insulin resistance (Roger et al, 2012). Altered glucose metabolism (absent diagnosed diabetes mellitus) has shown a significant correlation to atherosclerosis; control of hyperglycemia during coronary bypass surgery is an important strategy to reduce the risk of adverse clinical outcomes (Hillis et al, 2011; Nussmeier et al, 2009).
In addition to elevated lipid or cholesterol levels, inflammation increasingly has been implicated in the development of CAD. In particular, elevated homocysteine levels (which increase platelet aggregation) and high C-reactive protein (CRP) levels have been implicated (CRP is a biologic marker for inflammation and is associated with increased risk for CAD) (Horak et al, 2011). Additional recognized risk factors include metabolic syndrome (central obesity, hypertension, insulin resistance, and dyslipidemia) and obesity (Hillis et al, 2011; Roger et al, 2012). Hypertension and obesity increase the workload of the heart; obesity may also increase the risk for postoperative infection because adipose tissue vascularizes poorly. Additionally, a growing percentage of the population is aging. Age, in and of itself, rarely contraindicates intervention, but comorbidities often associated with older individuals constitute additional risk factors and areas—for example, cognitive impairment, depression, and decision-making capacity—that may require investigation (Chow et al, 2012).
Among the newer biomarkers denoting cardiovascular diseases are the natriuretic peptides—cardiac hormones synthesized and secreted by the atrium (atrial natriuretic peptide [ANP]) and the ventricle (B-type natriuretic peptide [BNP]). Discovered in 1981, natriuretic peptides have a regulatory effect that causes myocardial relaxation in response to acute increases in ventricular volume. Measurement of circulating peptides is increasingly used to identify heart failure and sudden cardiac death. Another biomarker under intensive study as a risk factor is lipoprotein(a) (Lp[a]), which has been identified from genetic studies as a causal link to MI; niacin has been shown to reduce the level of Lp(a) in some individuals (Hall, 2010).
Genetic risk factors for CAD and other cardiac disorders have been identified and are undergoing extensive study (Judge, 2012). Genetic studies have created a burgeoning body of knowledge about genetic mutations associated with risk factors such as CRP and possible pathways linking inflammatory processes with CAD (Kim et al, 2011; Roberts and Stewart, 2012a). Genetic risk variants mediate their risk in multiple ways; researchers have discovered that there are more unknown mechanisms than known mechanisms such as those associated with hypertension or lipids (Roberts and Stewart, 2012b). Soon an integral component of the preoperative assessment likely will include a genetic profile.
The risk for complications and major adverse cardiac events (MACEs) after cardiac surgery also appears to vary somewhat depending on gender (Hillis et al 2011; Newby and Douglas, 2012; Wenger, 2012). Investigations of risk factors in men and women for sternal wound infection have identified three significant risk factors that occur at significantly different rates in men and women: smoking, use of a single IMA, and age more than 70 years. Smoking and the use of a single IMA for bypass grafting are more common risk factors in men compared with women; women tend to be older than men at the time of surgery (Newby and Douglas, 2012). Fewer MACEs arose in women undergoing coronary bypass surgery without the use of cardiopulmonary bypass (CPB)—“off-pump” procedures—compared with surgery with CPB; however, malignant ventricular dysrhythmias (e.g., ventricular tachycardia), a calcified aorta, and preoperative renal failure are poor prognostic signs in women (Newby and Douglas, 2012). These findings should be incorporated into patient and family teaching plans with recommendations for lifestyle changes as indicated.
In addition to health status and risk factors, the perioperative nurse reviews patient medication history with particular attention to vasoactive drugs, anticoagulants, and other medications that can affect surgery. Nurses should note that patients taking aspirin and other antiplatelet drugs (such as 2b/IIIa inhibitors) may require intraoperative replacement of platelets. Lipid-lowering drugs (statins) have shown cardiovascular benefits, but in high doses (i.e., 80 mg, compared to moderate doses of 40 mg), they have been associated with new onset diabetes mellitus (Preiss et al, 2011). Patients taking herbal medicines may be at risk for increased bleeding, hypoglycemia, or other complications, depending on the specific side effects of some herbal drugs (see Chapter 30).
Patient knowledge and understanding of cardiovascular disease and related risk factors and their effect on functional, physiologic, and psychologic status should also be part of the perioperative nursing assessment. The patient’s personal strengths, external resources, and coping strategies are important subjects to consider. The perioperative nurse also notes any cultural, ethnic, spiritual, or religious beliefs that appear relevant to perioperative patient care.
Physical Examination.
Physical assessment provides the perioperative nurse with baseline information about potential problems that might require intervention. Normal, age-specific changes in very young and elderly populations must be differentiated from pathologic conditions. Chapters 26 and 27 provide extensive age-related considerations.
In addition to age-specific changes, a growing number of adults with acquired heart disease underwent surgery in childhood to repair congenital cardiac lesions (Webb et al, 2012). For example, patients with a previous Blalock-Taussig shunt for tetralogy of Fallot (see Chapter 26) may have altered pulmonary and subclavian artery anatomy. Likewise, patients with a mechanical closure device inserted for an atrial septal defect may be at risk for endocardial injury if the heart is retracted or otherwise manipulated too forcefully. The nurse must be aware that anatomic anomalies associated with the original congenital defect, or its subsequent repair, may mandate alteration of the current surgical plan. Consultation with the surgeon can alert the cardiac team to anticipate different anatomy (and surgical landmarks), special supplies and prosthetic materials, and potential complications.
Before beginning a review of systems, it is helpful to assess the patient’s functional capacity. How are activities of daily living accomplished? What are the barriers that make independent living difficult? How are activities performed when there are disabilities? What support systems are available? What educational resources are available? These and other questions will alert the clinician to the need for referrals and other resources to help the patient achieve optimal outcomes.
A systems review often starts with the skin. The appearance of the skin offers clues to cardiovascular status. Dryness, coolness, diaphoresis, paleness, edema, poor capillary refill, bruising, and petechiae may reflect impaired cardiovascular function. Visual problems and headaches may relate to inadequate cardiac output, atherosclerotic disease, peripheral vascular disease, aortic valve stenosis, or medications such as digitalis. The presence of chronic or local infection must be identified; if untreated, these are potential sources of postoperative infection. Nutritional status assessment (including metabolic syndrome) helps to determine increased risk for infection, skin breakdown, impaired wound healing, and other complications.
The patient’s level of consciousness, memory, comprehension, and emotional status require assessment. Confusion, restlessness, slurred speech, numbness, and paralysis may signal impaired perfusion. The perioperative nurse should note their presence preoperatively and communicate this to nurses receiving the patient postoperatively.
During respiratory assessment the perioperative nurse should note the use of accessory muscles or nostril flaring and should auscultate breath sounds. Adventitious sounds such as crackles and wheezes may point to pulmonary edema. Orthopnea, shortness of breath, or dyspnea may require elevation of the head of the stretcher and assistance during transfer onto the OR bed. If the patient is receiving oxygen, the flow rate and method of administration should be observed.
Alleviating pain is a prime consideration in the care of the cardiovascular patient because pain is a myocardial stressor. A patient with angina may arrive in the OR after nitroglycerin tablets have been administered or transdermal patches applied. Cold also increases the workload of the heart because the shivering that accompanies chilling elevates the metabolic rate; the patient should be kept warm.
Heart sounds, murmurs, and friction rubs provide clues to congenital, ischemic, valvular heart disease, or pericarditis. The patient may experience palpitations. Apical, radial, and femoral pulses also reflect cardiac function, and their rate, rhythm, and quality should be determined. The presence of cyanosis or peripheral edema should be noted.
Blood pressure is an important assessment consideration. The hypertensive patient may have left ventricular hypertrophy, and the hypotensive patient may display changes in neurologic, gastrointestinal, and renal function. Blood pressures should be checked bilaterally. Unequal pressures in the arms may be a contraindication for the use of the IMA as a bypass graft on the side of the lower blood pressure, where perfusion may not be optimal. Radial and ulnar artery pulses should be checked bilaterally when the radial artery is to be used as a bypass graft. Patients with dissections or aneurysms may have unequal carotid, femoral, brachial, or radial artery blood pressures when the lesion occludes one or more of these vascular branches.
Cardiac function affects all the body’s organ systems; therefore, assessment of the patient should be comprehensive whenever possible. A thorough assessment also alerts the physician and perioperative nurse to the need for special diagnostic tests and laboratory procedures.
Diagnostic Studies.
Most patients referred for surgery have had clinical evaluations, including invasive and noninvasive studies (Box 25-3). After the history and physical assessment, a resting electrocardiogram (ECG) follows. An exercise ECG (stress test) is often undertaken because ST-segment changes indicating myocardial ischemia may be apparent only during or after exercise. In patients with intractable dysrhythmias, electrophysiology (EP) studies may help locate irritable atrial or ventricular foci that can be surgically ablated, excised, or controlled with pharmacologic therapy. EP studies also may determine the need for internal defibrillators or antitachycardia devices. Bradycardia may indicate the need for pacemaker insertion.
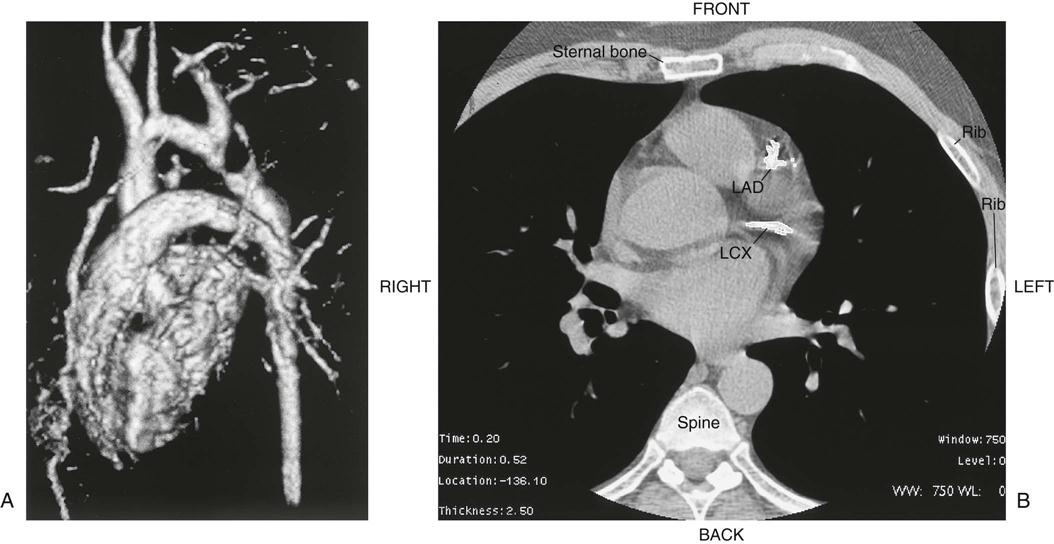
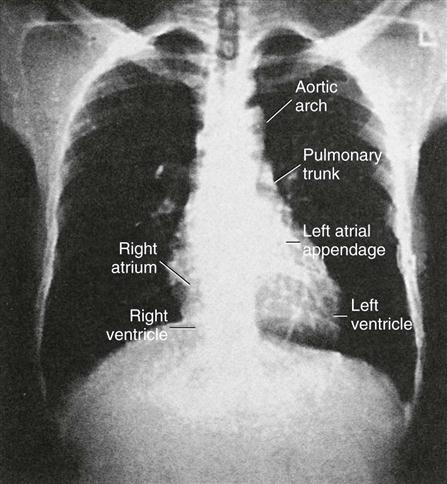
Chest radiography provides information about the size of the cardiac chambers, thoracic aorta, and pulmonary vasculature as well as the presence of calcium in valves, pericardium, coronary arteries, and aorta (Figure 25-8). Lateral chest radiographs of patients with prior sternal surgery show the chest wires and extent of pericardial adhesions. Magnetic resonance imaging (MRI) enables assessment of myocardial viability and also can image vascular structures with MRI angiograms that provide great clarity (Figure 25-9, A). In patients with suspected aortic or other vascular abnormalities, a computed tomography (CT) scan of the chest with intravenous injection of a contrast medium creates x-ray serial “slices” of the body area under study (see Figure 25-9, B). CT angiography is especially useful to image the aorta and the great vessels. CT images of the coronary arteries are used increasingly to identify areas of coronary calcification (shown in Figure 25-9, B), a recognized CV risk factor. One study demonstrated the value of coronary artery calcium scoring to identify patients who may be asymptomatic for CAD, but are at risk for increased mortality (Tota-Maharaj et al, 2012).
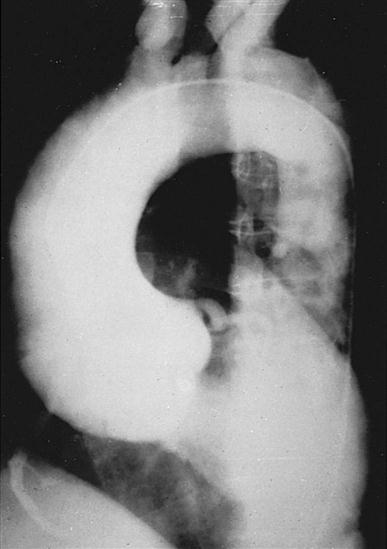
CT scans may be contraindicated in very unstable patients because their position in the tubelike scanner makes patient access difficult. Less frequently performed is arteriography with radiographic contrast dye to determine the size and location of the lesion and the site of the intimal tear in aortic dissections (Figure 25-10); digital subtraction angiography (DSA) provides clear images and requires less contrast material.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree