FIGURE 70-1 An ECG demonstrating atrial fibrillation with multiple QRS morphologies: biventricular paced and native IVCD with left axis deviation. Note the paced beats, marked with “![]() ” on the V1 rhythm strip, are characterized by prominent R wave in V1 and a narrower QRS compared to the native QRS.
” on the V1 rhythm strip, are characterized by prominent R wave in V1 and a narrower QRS compared to the native QRS.
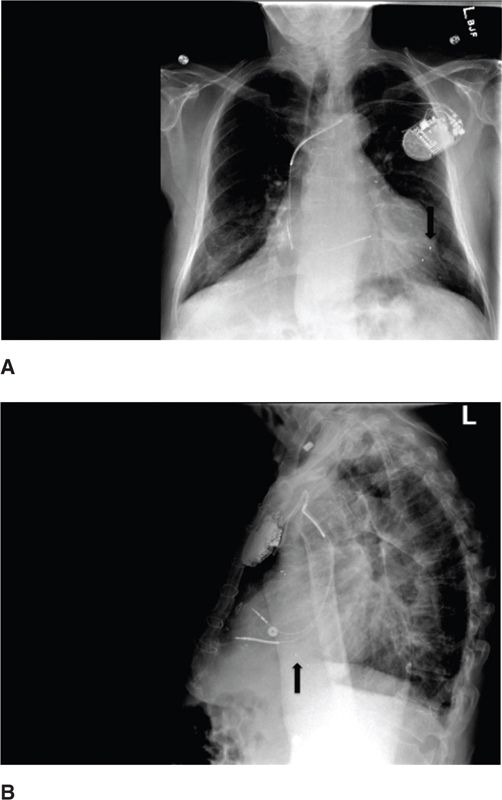
FIGURE 70-2 A posteroanterior and lateral radiograph demonstrating bilateral intersitial edema, cardiomegaly, and a standard 3-lead CRT device, with leads pacing the right atrium, right ventricle, and left ventricle. The LV lead is located in the coronary sinus, and the lead tip is position in the LV apex.
CRT: THE BASICS
CRT is an advanced pacing strategy able to restore electrical synchrony of both the atria and ventricles leading to improved chamber filling and pump function. Typical CRT systems include a right atrial lead, right ventricular lead, and left ventricular lead (Figure 70-2). The LV lead is typically implanted transvenously via the coronary sinus but can also be implanted epicardially via lateral thoracotomy. The 3-lead system allows for restoration of the AV and VV synchrony that is commonly lost in severe systolic heart failure with advanced conduction disease. Thus, CRT is more than simply biventricular pacing. CRT may be implanted with or without a defibrillator.
CRT has represented a significant advance in the treatment of severe symptomatic systolic heart failure with electrical dyssynchrony as evidenced by a prolonged QRS on the surface electrocardiogram. CRT has been associated with a significant reduction in heart failure symptoms, heart failure hospitalizations, and mortality1–4 and has become a widely accepted device therapy for a variety of indications. It has additionally been associated with improvements in exercise capacity, oxygen consumption, NYHA symptom class, and quality of life. While CRT is currently approved by the United States Food and Drug Administration for patients with EF <35% and either 1) QRS >120 ms and NYHA class III/IV symptoms or 2) QRS >130 ms, LBBB, and NYHA II symptoms, major society guidelines define additional appropriate patient subsets in whom the use of CRT is thought to be of benefit.5 These subsets are largely dictated by the width of the QRS interval and morphology of the conduction defect. In most situations, CRT is considered appropriate for patients with a LBBB and a QRS width ≥120 ms, while in patients with a non-LBBB morphology, the QRS width should ideally be ≥150 ms.
IMPACT ON MYOCARDIAL STRUCTURE AND FUNCTION
CRT has been clearly linked to favorable changes in myocardial structural and functional parameters, termed reverse remodeling. Ongoing therapy has been associated with increased ejection fraction, reduced mitral regurgitation, and decreased chamber size, including LV dimension and volume. Patients manifesting these changes are often referred to as echocardiographic responders. Notably, some degree of echocardiographic response often occurs immediately after initiation of CRT, though the maximal extent of response typically requires approximately 6 months of therapy. Echocardiographic response is tightly linked to improvements in clinical outcomes and is felt to be an important mechanism underlying the clinical improvements afforded by CRT.6
IMPACT ON ELECTRICAL FUNCTION AND DYSFUNCTION
The initiation of CRT is associated with a number of changes in electrical activation, function, and stability. Simultaneous or near simultaneous depolarization of the ventricles via biventricular pacing decreases the electrical dyssnchrony associated with ventricular conduction delay, leading to an increasingly synchronous mechanical contraction. Favorable change in the ventricular activation sequence may be manifested by a narrowing of the QRS interval with biventricular pacing. CRT additionally involves optimization of the AV interval, allowing for improvements in diastolic filling with a reduction in diastolic mitral regurgitation. AV optimization often involves programming to reduce the AV interval, which is frequently prolonged in the context of cardiomyopathy.
Initial reports suggested that CRT might be associated with an increase in ventricular arrhythmias. Multiple mechanisms were postulated, including epicardial pacing leading to a reversal of the transmural gradient with increased heterogeneity of repolarization, functional reentry, and torsades de pointes, and biventricular pacing causing a collision of multiple wavefronts in close proximity to a susceptible anatomic substrate. While these mechanisms may lead to arrhythmogenesis in a minority of CRT patients, antiarrhythmic mechanisms typically predominate, outweighing the theoretical deleterious effects. The antiarrhythmic effects of CRT are likely related to favorable changes in wall tension, LV size, neurohormonal activation, LV mass, and oxygen consumption, which are likely important for arrhythmogenesis. CRT additionally decreases pauses and conduction delays, which are important mechanisms for pause dependent and macroreentrant arrhythmias, respectively. Thus, while CRT may exert a number of pro- and antiarrhythmic effects on the myocardium, it is now well accepted that the net effect of CRT is a reduction of ventricular arrhythmias and sudden death. Improvements in electrical stability have been linked with echocardiographic response. Some have suggested that the antiarrhyhmic effect of CRT is sufficient to preclude benefit from concomitant ICD implantation, though this remains controversial, and most CRT patients typically receive an ICD.
FOLLOW-UP AND MONITORING RESPONSE TO THERAPY
The relation between echocardiographic response and improved clinical outcomes has led clinicians to assess routinely response to CRT with an echocardiogram at approximately 6 months after implant, as a supplement to history, physical, and NYHA class assessment. By 6 months, approximately two-thirds of patients experience an improvement in EF, a decrease in LV end systolic volume, and/or class improvement in NYHA symptom class. Those who do not demonstrate improvement based on one or more of these metrics are referred to as nonresponders. Of note, although the term “nonresponder” is a frequently used classification in clinical practice and research, there is not a single agreed upon definition. The patient described in the clinical vignette would be considered a nonresponder based on lack of symptomatic improvement and decrement in EF. Though heterogeneity in the definitions of responder and nonresponder exist, it remains a clinically useful concept as changes in EF, LV end systolic volume, and symptom class provide important prognostic information regarding the success of CRT. Based on this, all patients should undergo complete assessment including echocardiogram and NYHA symptom class assessment at baseline and 6 months after implantation to assess responder status. It should be noted that more frequent clinical and echocardiographic assessment might be warranted for early detection of patients who are at risk of nonresponse.
The identification of CRT nonresponse should prompt a comprehensive evaluation for reversible causes. CRT nonresponse may be due to one or more of a number of factors, including suboptimal AV and/or VV optimization, anemia, arrhythmia, insufficient biventricular pacing, suboptimal lead positioning, suboptimal medical therapy, narrow QRS, and patient noncompliance. A comprehensive examination should include device interrogation, ECG, basic laboratory tests, PA and lateral chest x-ray, and echocardiogram with a trial of different AV and VV device settings. Multidisciplinary evaluation from electrophysiology, heart failure, and cardiac imaging perspectives should strongly be considered for all nonresponders.7,8 Therapy for nonresponders may include medication adjustment, device optimization, lead revision, catheter ablation, antiarrhythmic drugs, and cardioversion.
DETERMINANTS OF RESPONSE
Despite ongoing advances in CRT, nearly one-third of patients are nonresponders due to patient selection and/or device related issues. Characteristics commonly associated with increased risk of nonresponse include male sex, ischemic cardiomyopathy, non-LBBB morphology, narrow QRS, atrial fibrillation, low percentage of biventricular pacing, and suboptimal lead position.
QRS Duration
Although advanced conduction disease is typically associated with worsened cardiovascular disease and worsened outcomes in many populations, a prolonged QRS interval is associated with improved outcomes among those undergoing CRT.9 This seemingly paradoxical finding is related to the fact that those with heart failure and a prolonged QRS frequently have heart failure at least in part due to conduction disease and resultant electrical dyssynchrony. Thus, a prolonged QRS represents an “electrical problem” that has the capacity to be treated by an electrical therapy (eg, CRT). Conversely, patients with severe heart failure and a narrower QRS are less likely to have an “electrical problem” underpinning pump dysfunction, and thus are less likely to respond to this pacing therapy.10,11
Bundle Branch Morphology
Though QRS duration has emerged as an important determinant of response, a number of recent studies have strongly suggested that QRS morphology is at least as important.12–14
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree