Figure 14-1. Action Potential for Atrial and Ventricular Tissue
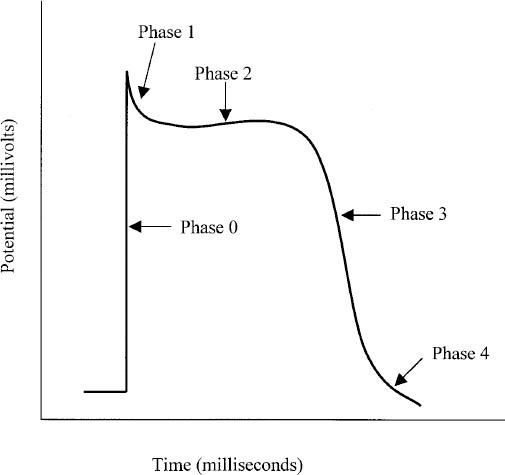
■ The absolute refractory period is the time during which cardiac cells cannot conduct or propagate an action potential (Figure 14-1 and Table 14-1).
■ The relative refractory period is the time during which cardiac cells may conduct and propagate action potentials secondary to strong electrical stimuli.
Normal Conduction System
The sinoatrial (SA) node, located in the right atrium, initiates an impulse that
■ Stimulates the left atrium and atrioventricular (AV) node, which
■ Stimulates the left and right bundle branches via the bundle of His, which then
■ Stimulates Purkinje fibers and causes ventricular contraction.
Table 14-1. Phases of Atrial and Ventricular Tissue Action Potential

14-4. Mechanisms of Arrhythmia
Cardiac arrhythmias arise secondary to the following disorders:
■ Automaticity (impulse generation)
■ Latent pacemaker (non-SA node pacemaker)
■ Triggered automaticity (early or late after-depolarizations)
■ Reentry
■ Impulse conduction
■ Automaticity and impulse conduction
14-5. Clinical Manifestations
Symptoms
Symptoms associated with ventricular arrhythmias range from asymptomatic to loss of consciousness and death. Patients with ventricular tachycardia (VT) may be asymptomatic, but VT can result in hypotension, syncope, or death. Ventricular fibrillation produces no cardiac output and causes most cases of sudden cardiac death.
Both bradyarrhythmias and tachyarrhythmias can be associated with reduced cardiac output, producing symptoms that include dizziness, syncope, chest pain, fatigue, confusion, and exacerbation of heart failure. Patients with tachyarrhythmias may report palpitations. With atrial fibrillation or flutter, patients may also experience dizziness, palpitations, light-headedness, dyspnea, and worsening heart failure, as well as symptoms of transient ischemic attack (TIA) or stroke.
Signs
■ Electrocardiogram (ECG) abnormalities may be present.
■ Ventricular rate can be assessed by documenting the heart rate from the radial artery or by carotid palpation.
14-6. Diagnostic Criteria and Therapy According to Arrhythmia Classification
Arrhythmias are defined by the following:
■ Anatomic location
• Supraventricular arrhythmias arise from abnormalities in the SA node, the atrial tissue, the AV node, or the bundle of His.
• Ventricular arrhythmias originate from below the bundle of His.
■ Ventricular rate
• Bradyarrhythmias: Heart rate < 60 beats per minute (bpm).
• Tachyarrhythmias: Heart rate > 100 bpm.
Bradyarrhythmias
Sinus bradycardia
Diagnostic criteria and characteristics
Heart rate is less than 60 bpm; otherwise, the ECG is normal.
Mechanism of arrhythmia
The mechanism of arrhythmia is decreased SA node automaticity.
Clinical etiology
Causes include acute myocardial infarction (MI); hypothyroidism; drug-induced causes (β-blockers including ophthalmic agents, digoxin, calcium channel blockers [diltiazem, verapamil], clonidine, amiodarone, and cholinergic agents); and hyperkalemia.
Treatment goals
Restore normal sinus rhythm if the patient is clinically symptomatic.
Drug and nondrug therapy
For intermittent symptomatic episodes: administer atropine 0.5–1 mg intravenously, repeated up to maximum dose of 3 mg.
For persistent episodes or if there is no response to atropine, place transvenous or transcutaneous pacemaker.
Atrioventricular block
Diagnostic criteria and characteristics
Criteria are as follows:
■ First-degree: Prolonged PR interval > 0.20 seconds, 1:1 atrioventricular conduction
■ Second-degree Mobitz type I: Gradual prolongation of PR interval followed by P wave without ventricular conduction
■ Second-degree Mobitz type II: Constant PR interval with intermittent P wave without ventricular conduction; may have widened QRS complex
■ Third-degree: Heart rate 30–60 bpm; no temporal relation between atrial and ventricular contraction; ventricular contraction initiated by AV junction or ventricular tissue
Mechanism of arrhythmia
The mechanism of arrhythmia is prolonged conduction.
Clinical etiology
Causes include AV nodal disease; acute MI; myocarditis; increased vagal tone; drug-induced causes (β-blockers, digoxin, calcium channel blockers [diltiazem, verapamil]; clonidine, amiodarone, cholinergic agents); and hyperkalemia.
Treatment goals
Restore sinus rhythm if the patient is symptomatic.
Drug and nondrug therapy
If the cause is reversible, treat with a temporary pacemaker or intermittent atropine.
If the condition is chronic, implant a permanent pacemaker.
Supraventricular Arrhythmias
Atrial fibrillation and atrial flutter
Diagnostic criteria and characteristics
Criteria are as follows:
■ Atrial fibrillation: No P waves; irregularly irregular QRS pattern
■ Atrial flutter: Sawtooth P wave pattern; regular QRS pattern
■ Ventricular response: Usually fast but can also be slow or normal
Mechanism of arrhythmia
The mechanism of arrhythmia is enhanced automaticity and reentry.
Clinical etiology
Causes include rheumatic heart disease, heart failure, hypertension, ischemic heart disease, diabetes, obesity, obstructive sleep apnea, pericarditis, cardiomyopathy, mitral valve prolapse, cardiac surgery, infection, alcohol abuse, hyperthyroidism, chronic obstructive pulmonary disease, pulmonary embolism, and idiopathic causes (lone atrial fibrillation).
Atrial fibrillation and flutter are the most commonly occurring arrhythmias, and risk increases with age.
Complications include stroke and heart failure exacerbation.
Specific treatment goals for atrial fibrillation
Figure 14-2 illustrates a treatment algorithm for atrial fibrillation.
Control the ventricular rate
Digoxin can be useful in patients with left ventricular systolic dysfunction (e.g., left ventricular ejection fraction < 40%), especially when combined with a β-blocker; digoxin slows the ventricular rate but has poor control in hyperadrenergic-induced atrial fibrillation.
The most effective agents are β-blockers (esmolol, metoprolol, propranolol, others) and calcium channel blockers (diltiazem, verapamil). Calcium channel blockers should not be used in patients with left ventricular systolic dysfunction.
For rapid control of ventricular rate, the intravenous (IV) route of administration should be used.
Digoxin, calcium channel blockers, and β-blockers do not restore sinus rhythm.
The target resting heart rate remains controversial, but current guidelines recommend a resting rate of < 80–110 bpm.
Restore and maintain sinus rhythm
Acute conversion to sinus rhythm may be required in patients with atrial fibrillation who are hemodynamically unstable (e.g., hypotensive).
Restoration of sinus rhythm is usually accomplished by electrical cardioversion or administration of antiarrhythmic drugs (AADs).
An important area of controversy centers on whether chronic AAD therapy should be administered to maintain sinus rhythm after cardioversion (rhythm control approach) or whether patients should simply be treated with agents to control ventricular response and anticoagulants to prevent thromboembolic stroke (rate control approach).
Historically, AADs were frequently used to restore and maintain sinus rhythm in patients with atrial fibrillation (rhythm control approach). With chronic therapy, AADs approximately double the chances of a patient remaining in sinus rhythm. However, this approach exposes patients to the large number of adverse effects associated with AADs. The rationale for this approach includes the possibility of fewer symptoms, lower risk of stroke, improved quality of life, and reduced mortality. Nevertheless, these benefits have never been proven in large clinical trials.
The alternative approach, so-called rate control, involves using drugs to control the ventricular response and chronic anticoagulation for stroke prevention.
The rate control and rhythm control approaches have recently been compared in a number of large clinical trials, and the studies demonstrate no advantage for rhythm control over rate control for improving cardiovascular outcomes. Rhythm control is reasonable to consider with patients in whom the ventricular rate cannot be controlled or with those who continue to have symptoms despite adequate control of ventricular response. The current American Heart Association–American College of Cardiology guideline recommendations for the use of AADs to maintain sinus rhythm can be found at http://circ.ahajournals.org/content/early/2014/04/10/CIR.0000000000000041.citation. Regardless of the approach, appropriate anticoagulation based on the presence of stroke risk factors is needed.
Figure 14-2. Treatment Algorithm for Atrial Fibrillationa
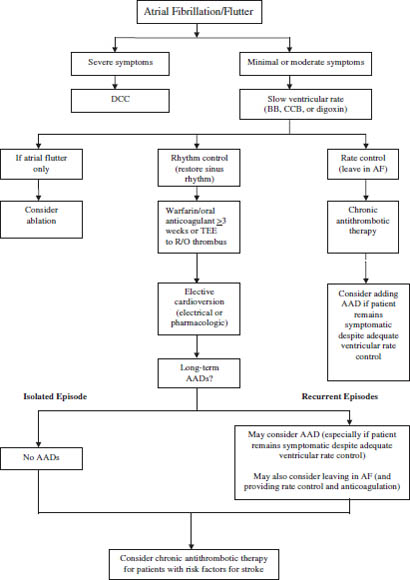
Reproduced with permission from Sanoski, Bauman, 2011.
AADs, antiarrhythmic drugs; AF, atrial fibrillation; BB, β-blocker; CCB, nondihydropyridine calcium channel blocker; DCC, direct-current cardioversion; R/O, rule out; TEE, transesophageal echocardiography.
a. Selection of the most appropriate antithrombotic therapy is based on the presence of risk factors for stroke, regardless of whether the rhythm or rate control approach is selected.
Even when chronic antiarrhythmic therapy is used to maintain sinus rhythm, it is not 100% effective. Therefore, this approach is usually reserved for patients with recurrent, symptomatic episodes.
Nonpharmacologic therapy, particularly catheter ablation to abolish the reentrant focus causing atrial fibrillation, is now being used frequently.
Prevent thromboembolism
Prior to use of pharmacologic or direct-current cardioversion: If atrial fibrillation is present for ≥ 48 hours or of an unknown duration, anticoagulate with warfarin (INR [international normalized ratio] 2–3), dabigatran, rivaroxaban, or apixaban for at least 3 weeks prior to cardioversion, and continue for at least 4 weeks after sinus rhythm has been restored.
If atrial fibrillation is present for ≥ 48 hours or of an unknown duration and there is no anticoagulation in the preceding 3 weeks, transesophageal echocardiography (TEE) is often used to determine the presence of atrial thrombus. If no thrombus is seen, cardioversion can be attempted provided anticoagulation is achieved before TEE and continued for at least 4 weeks afterward. If atrial thrombus is seen on TEE, anticoagulation should be initiated and a repeat TEE performed before attempting later cardioversion.
Recommended chronic antithrombotic therapy
The choice of the optimal antithrombotic agent is based on the patient-specific risks of stroke and bleeding and should also involve shared decision making with patients based on their values and preferences. Current guidelines now recommend the use of the CHA2DS2-VASc score to calculate stroke risk in patients with nonvalvular atrial fibrillation instead of the CHADS2 score, although some clinicians continue to use the CHADS2. Each stroke risk factor is assigned a point value, and the total number of points is associated with an annual risk of stroke. The CHA2DS2-VASc risk factors for stroke are as follows:
■ Congestive heart failure or impaired left ventricular systolic function = 1 point
■ Hypertension = 1 point
■ Age ≥ 75 years = 2 points
■ Diabetes = 1 point
■ Stroke/TIA/thromboembolism = 2 points
■ Vascular disease (MI, peripheral artery disease, or aortic plaque) = 1 point
■ Age 65–74 years = 1 point
■ Sex category (female) = 1 point
The treatments below are recommended according to a CHA2DS2-VASc score:
■ CHA2DS2-VASc score = 0: omitting antithrombotic therapy is reasonable.
■ CHA2DS2-VASc score = 1: no antithrombotic therapy or treatment with oral anticoagulation (warfarin INR 2–3, dabigatran, rivaroxaban, or apixaban) or aspirin can be considered.
■ CHA2DS2-VASc score ≥ 2: oral anticoagulation (warfarin INR 2–3, dabigatran, rivaroxaban, or apixaban) is recommended.
■ For patients undergoing coronary revascularization (e.g., drug-eluting stent) with atrial fibrillation and a CHA2DS2-VASc score ≥ 2, the use of clopidogrel 75 mg daily together with oral anticoagulants but without aspirin may be reasonable.
■ For patients with atrial fibrillation that have mechanical heart valves, warfarin therapy is recommended with an INR target based on the type and location of the prosthetic valve.
■ Dabigatran and rivaroxaban are not recommended for patients who have atrial fibrillation and end-stage chronic kidney disease or who are on hemodialysis.
■ Dabigatran, rivaroxaban, or apixaban should not be used in patients with atrial fibrillation and a prosthetic heart valve.
Warfarin (Coumadin)
Dosage forms
■ Tablets: 1 mg (pink), 2 mg (lavender), 2.5 mg (green), 3 mg (tan), 4 mg (blue), 5 mg (peach), 6 mg (teal), 7.5 mg (yellow), 10 mg (white)
■ Injections (IV): 5 mg powder for reconstitution (2 mg/mL)
Mechanism of action
Warfarin inhibits vitamin K epoxide-reductase and vitamin K reductase, preventing the conversion of vitamin K epoxide to vitamin K. It ultimately inhibits formation of vitamin K–dependent coagulation factors II, VII, IX, and X, as well as proteins C and S.
Absorption
Bioavailability of warfarin is 80–100% following oral administration. It is absorbed in the upper gastrointestinal tract. Food or enteral feedings may decrease the rate and extent of absorption.
Distribution
Warfarin is 99–99.5% protein bound, primarily to albumin.
Metabolism and elimination
Warfarin is administered as a racemic mixture of S- and R-warfarin; the S-isomer is five times more potent than the R-isomer. The S-isomer is primarily metabolized in the liver via cytochrome P450 2C9 (CYP2C9). The R-isomer is metabolized by several other enzymes of the cytochrome P450 system.
Warfarin has low-extraction pharmacokinetic characteristics.
Clearance decreases with increasing age.
The half-life of the R-isomer is 45 hours; for the S-isomer, it is 33 hours.
Pharmacogenomics
Recent studies show that genetic polymorphisms can markedly influence the metabolism and response to warfarin. Mutations in two genes—CYP2C9, which codes for the hepatic enzyme that metabolizes S-warfarin, and VKORC1, which regulates the vitamin K epoxide-reductase enzyme—can account for up to 50% of the variability in the dose of warfarin. The current package insert contains information regarding altered responses caused by polymorphisms in the CYP2C9 and VKORC1 genes. However, the use of genetic testing to prospectively determine the dose of warfarin remains controversial.
Pharmacodynamics
The S-isomer is approximately five times more potent than the R-isomer in inhibiting vitamin K reductase. Its pharmacodynamic effect (change in INR) is an indirect effect of the decreased formation of the vitamin K–dependent coagulation factors II, VII, IX, and X. The long half-lives of these factors result in delayed onset of action and delayed response to dosage changes.
Adverse effects
Several adverse effects are possible:
■ Bleeding can occur, roughly proportional to the degree of anticoagulation.
■ Skin necrosis, related to depletion or deficiency of protein C, is possible. This effect usually occurs within 10 days of warfarin initiation. Incidence is low.
■ Purple-toe syndrome may occur. This syndrome usually occurs 3–8 weeks after warfarin initiation. Incidence is low.
■ Birth defects and fetal hemorrhage are possible. Therefore, warfarin is pregnancy category X.
Some common drug–drug interactions
Medications decreasing warfarin anticoagulant response are as follows:
■ Barbiturates
■ Carbamazepine
■ Cholestyramine
■ Griseofulvin
■ Nafcillin
■ Phenytoin (chronic therapy)
■ Rifampin
Medications that can increase warfarin anticoagulant response include the following (not a complete list):
■ Acetaminophen
■ Allopurinol
■ Amiodarone
■ Azole antifungal agents
■ Cimetidine
■ Ciprofloxacin, levofloxacin
■ Diltiazem
■ Erythromycin, clarithromycin
■ Fenofibrate
■ Fish oil
■ Metronidazole
■ Omeprazole (R-enantiomer)
■ Phenytoin (acute therapy)
■ Propafenone
■ Simvastatin, fluvastatin
■ Sulfinpyrazone
■ Trimethoprim-sulfamethoxazole
Dosing management
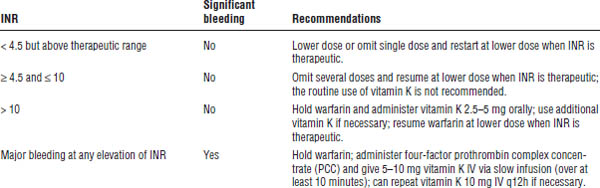
Patients should take a once-daily dose of 1–10 mg orally. Patient response is highly variable. Management of elevated INR is described in Table 14-2.
Monitoring
The standard for assessing the degree of anticoagulation is the INR = (observed prothrombin ratio)ISI, where ISI is the International Sensitivity Index, which corrects for variability in thromboplastin sensitivity.
Initially, the INR is monitored every 1–2 days until the desired INR is achieved and has stabilized at a given dose. Periodic INR monitoring (i.e., monthly) is recommended thereafter unless dosage changes are made.
Dabigatran (Pradaxa)
Dabigatran etexilate is a pro-drug (inactive) that after oral administration is converted to the active form dabigatran, which exerts its anticoagulant effect by direct inhibition of thrombin. It was recently approved by the U.S. Food and Drug Administration (FDA) to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. The medication guide enclosed in the packaging should always be given to the patient each time dabigatran is dispensed.
Table 14-2. Management of Elevated International Normalized Ratio
Dosage forms
Capsules: 75, 150 mg
Mechanism of action
Dabigatran is a competitive reversible direct inhibitor of thrombin (factor IIa). By inhibiting thrombin, dabigatran blocks the conversion of fibrinogen to fibrin, thus preventing thrombus development.
Absorption
Bioavailability of dabigatran following oral administration of dabigatran etexilate is 3–7%. It may be administered with or without food. The capsules should not be broken, chewed, or opened before administration because this may result in a significant increase in bioavailability. Dabigatran is a substrate of the drug efflux transporter P-glycoprotein (P-gp).
Distribution
Dabigatran is approximately 35% protein bound.
Metabolism and elimination
The inactive dabigatran etexilate ester is hydrolyzed by esterases in the gut and liver to the active dabigatran moiety. Dabigatran then undergoes glucuronidation to several metabolites that are active. Dabigatran is primarily (80%) eliminated in the urine. The dose of dabigatran for patients with a creatinine clearance of > 30 mL/min is 150 mg orally twice daily. The dose should be reduced to 75 mg twice daily in patients with a creatinine clearance of 15–30 mL/min. Dabigatran is not a substrate, inhibitor, or inducer of cytochrome P450 enzymes. The half-life ranges from 12 to 17 hours.
Pharmacodynamics
Dabigatran prolongs the activated partial thromboplastin time (aPTT), the thrombin clotting time (TT), and the ecarin clotting time. At clinically used doses, dabigatran has little effect on the INR. However, no clinical tests are used to monitor the intensity of anticoagulation with dabigatran.
Adverse effects
■ The risk of bleeding is increased in patients receiving dabigatran. Unlike warfarin, no specific agent is available to reverse dabigatran’s anticoagulant effect.
■ Gastrointestinal adverse reactions including dyspepsia, nausea, abdominal pain or discomfort, or gastroesophageal reflux disease (GERD) can occur in up to 35% of patients.
Drug–drug interactions
The exposure to dabigatran is significantly reduced when administered with rifampin, a potent P-gp inducer. Therefore, the concomitant use of dabigatran with rifampin or other P-gp inducers should be avoided.
Dabigatran does not require dosage adjustment when administered with P-gp inhibitors such as ketoconazole, verapamil, amiodarone, quinidine, and clarithromycin. However, in patients with creatinine clearance of 15–30 mL/min, dabigatran should not be used in combination with P-gp inhibitors.
Monitoring
Unlike warfarin, in which close monitoring of the INR is required, with dabigatran no laboratory monitoring of the intensity of anticoagulation is required.
Rivaroxaban (Xarelto)
Rivaroxaban (Xarelto) is a factor Xa inhibitor recently approved by the FDA to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Although approved for this indication, its use is not included in current guidelines for antithrombotic therapy of atrial fibrillation. The medication guide enclosed in the packaging should always be given to the patient each time rivaroxaban is dispensed.
Dosage forms
Tablets: 10, 15, 20 mg
Mechanism of action
Rivaroxaban is a factor Xa inhibitor that selectively blocks the factor Xa active site and does not require a cofactor (e.g., antithrombin III) for activity. Activation of factor X to factor Xa plays an important role in the clotting cascade.
Absorption
Bioavailability of the rivaroxaban 20 mg dose is 66% in the fasting state. Co-administration with food increases bioavailability by approximately 40–75%. Rivaroxaban should be administered with food at the evening meal.
Distribution
Rivaroxaban is approximately 92–95% protein bound.
Metabolism and elimination
Approximately 40–45% of rivaroxaban is eliminated unchanged by the kidneys. It also undergoes oxidative metabolism by CYP3A4 and possibly other cytochrome P450 enzymes. The dose of rivaroxaban for patients with a creatinine clearance of > 50 mL/min is 20 mg orally once daily with the evening meal. The dose should be reduced to 15 mg once daily in patients with a creatinine clearance of 15–50 mL/min. Rivaroxaban should not be used in patients with a creatinine clearance of < 15 mL/min. The drug does not inhibit or induce cytochrome P450 enzymes or drug transporters. Rivaroxaban is transported by P-gp. The half-life ranges from 5 to 9 hours.
Pharmacodynamics
Rivaroxaban produces dose-dependent inhibition of factor Xa activity. It also prolongs the aPTT and the prothrombin time (PT). However, no clinical tests are used to monitor the intensity of anticoagulation with rivaroxaban.
Adverse effects
■ The risk of bleeding is increased in patients receiving rivaroxaban.
■ Discontinuing rivaroxaban places patients at increased risk of thromboembolic events. If rivaroxaban is discontinued for reasons other than bleeding, administration of another anticoagulant should be considered.
■ Epidural and spinal hematomas can occur in patients receiving rivaroxaban who undergo neuraxial anesthesia or spinal puncture. These hematomas can result in long-term or permanent paralysis.
■ Other adverse reactions include extremity pain, muscle spasm, syncope, and pruritus.
Drug–drug interactions
Rivaroxaban should not be administered with agents that are combined P-gp and strong CYP3A4 inhibitors (e.g., ketoconazole, fluconazole, itraconazole, ritonavir, clarithromycin, erythromycin).
Rivaroxaban should not be administered with agents that are combined P-gp and strong CYP3A4 inducers (carbamazepine, phenytoin, rifampin, St. John’s wort).
Monitoring
Unlike warfarin, in which close monitoring of the INR is required, no laboratory monitoring of the intensity of anticoagulation with rivaroxaban is required.
Apixaban (Eliquis)
Apixaban (Eliquis) is a factor Xa inhibitor indicated to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and for the prophylaxis of deep vein thrombosis in patients who have undergone hip or knee replacement surgery. The medication guide enclosed in the packaging should always be given to the patient each time apixaban is dispensed.
Dosage forms
Tablets: 2.5, 5 mg
Mechanism of action
Apixaban is a factor Xa inhibitor that selectively blocks the factor Xa active site and does not require a cofactor (e.g., antithrombin III) for activity. Activation of factor X to factor Xa plays an important role in the clotting cascade.
Absorption
Bioavailability of apixaban is approximately 50% and is not affected by food.
Distribution
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


