and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
10.1 Definition, Site and Incidence
The development of malignancy from a pleomorphic adenoma is well recognised but is considerably more complex than previously thought. Apart from tumours with obvious histological malignancy, there are some pleomorphic adenomas that have atypical histological features only and yet others with malignant biological behaviour but with benign histology, the so-called metastasising pleomorphic adenomas (see Chap. 3). Carcinomas ex pleomorphic adenoma are also subdivided into intracapsular (non-invasive, in situ) carcinoma, minimal invasive carcinoma and invasive carcinoma. Poorly differentiated adenocarcinoma not otherwise specified, salivary duct carcinoma and adenoid cystic carcinoma are the most commonly reported histological types of malignancy [35, 54]. Virtually any salivary type carcinoma may develop; however, with the possible exception of acinic cell carcinoma. Usually only one histological type is encountered [4, 11, 15, 17, 28, 29, 32, 38, 39, 48, 49, 53, 56, 66, 72, 74, 77]. On very rare occasions, carcinosarcoma or non-salivary tumours such as malignant rhabdoid tumours may arise [29]. In his extensive review of the literature, Gnepp culled 42 cases of carcinosarcoma and in at least 11 of these cases there was a previous history of pleomorphic adenoma [32].
Malignant transformation occurs in 5–15 % of pleomorphic adenomas and most often in long-standing tumours with multiple recurrences. The tumours have been present for long periods of time and in some series as long as 23 years [7]. In other series, however, 45 % of tumours had been present less than 3 years [51]. Many larger series of carcinoma ex pleomorphic adenoma (CXPA) have been published and indicate that CXPA constitutes approximately 12 % of all malignant salivary tumours (range 2.8–42.2 %). The annual incidence rate has been given to 0.17 tumours per one million persons [32, 35]. These data support that CXPA is the fourth most common salivary malignancy. Already 30 years ago, Livolsi and Perzin emphasised that CXPAs are not rare lesions and constituted 15 % of malignant salivary gland tumours in their institution [51]. In a large series of 741 salivary gland tumours diagnosed in a UK population over 30 years, however, Jones and associates found polymorphous low-grade adenocarcinoma to be the fourth most common malignancy (10.8 %), followed by CXPA (9 %). Their finding of a higher incidence of polymorphous low-grade adenocarcinoma than CXPA is likely explained by that the material from the first two decades of their study was predominantly of minor salivary gland origin [41]. CXPA tends to present late in life, often in sixth and seventh decades, which is a decade later than pleomorphic adenoma. CXPA is exceptional in children and teenagers, and in a series of 326 patients with CXPA, only four were under the age of 20 [33].
CXPA most commonly arises in the parotid gland and only rarely in the submandibular gland [35, 37, 39, 45, 85]. Cases have been reported from intraoral minor salivary glands, particularly palatal glands [12, 16, 46, 88]. In the series of 146 cases of CXPA reported by Spiro and associates [75], approximately 73 % arose in the parotid glands, 16 % in the submandibular gland, 11 % in intraoral minor salivary gland and one case only in the sublingual gland. Similar figures are given in the review by Gnepp [32] where 82 % of tumours were in major salivary glands and 18 % in minor salivary glands. It also occurs in the lacrimal gland [9, 40, 47, 65, 71, 84]. It is exceedingly rare elsewhere but has been described in the sublingual gland [3], trachea [19, 20], buccal mucosa [59, 69], lips [57], sinonasal tract [14, 82], skin [68, 73] and also nasopharyngeal tumours have been reported [30, 82].
10.2 Clinical Features and Gross Appearances
Carcinoma ex pleomorphic adenoma frequently presents as a painless mass and in some series inasmuch as 55–80 % of patients. Pain is however sometimes a presenting symptom as well as facial nerve palsy that occurs in approximately a third of patients [32, 51, 75]. They arise in a pleomorphic adenoma and more frequently so in recurrent pleomorphic adenomas. Pleomorphic adenoma has an average size of 2–6 cm but CXPAs are considerably larger and are reported to have an average size that is double that of pleomorphic adenoma. However, in the series of 73 cases of CXPAs reported by Lewis et al. [49], the mean overall size was 3.9 cm. Typically the patients present with a long-standing parotid mass (several years) that suddenly has increased considerably in size. The tumours are macroscopically usually poorly circumscribed, and cut surface often shows haemorrhagic and necrotic areas. They are usually readily recognised as a malignancy [10, 27, 32, 51, 83] (Fig. 10.1).
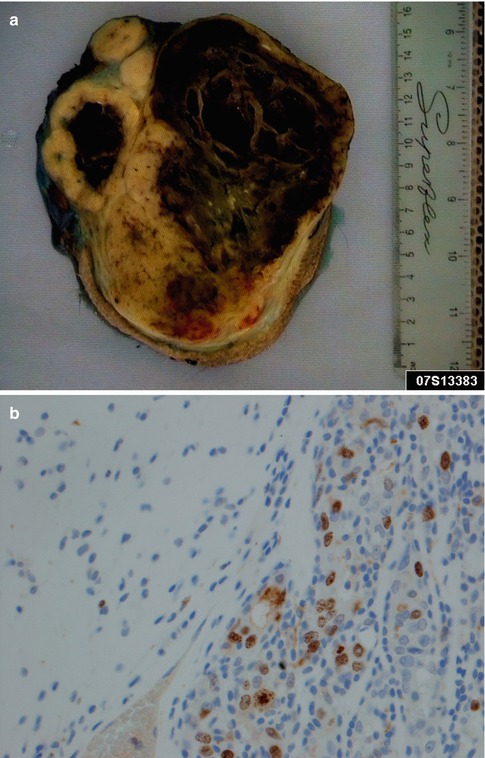

Fig. 10.1
(a) A large parotid carcinoma ex pleomorphic adenoma, partly cystic and with solid white areas with haemorrhage. Microscopy showed only smaller areas with remnants of benign pleomorphic adenoma. (b) High Ki-67 index in the carcinoma (right) compared to the pleomorphic adenoma (left)
10.3 Histopathology
The diagnosis of CXPA requires a histological malignant epithelial component and remnants of a benign pleomorphic adenoma. The proportion of the two components varies considerably. In some cases, the majority of the tumour is composed of benign pleomorphic adenoma with only a small area containing carcinoma. In other tumours only small foci of residual benign tumour is present, and extensive sampling may be required needed so to identify the benign component. Sometimes remnants of the pleomorphic adenoma cannot be found, but if there is clinicopathological documentation (history of swelling is not enough) of a previous pleomorphic adenoma on the same anatomical location, still the histological classification of CXPA can be made [35]. CXPAs should further be subclassified into non-invasive (intracapsular, in situ), minimally invasive and widely invasive carcinomas. The rationale behind this is that tumours belonging to the first two groups have an excellent prognosis and apparently need no adjuvant treatment to radical surgery. Tumours where the malignant component shows no invasion outside the capsule of the pleomorphic adenoma are classified as non-invasive (intracapsular, in situ) CXPA. There are also the odd reports of intracapsular CXPA with lung metastases that were composed exclusively of benign PA elements, hence further indicating a continuum between the so-called metastasising pleomorphic adenoma and CXPA (see Chaps. 3 and 15). In cases of minimally invasive CXPA, the extension of the invasion outside the capsule is limited to ≤1.5 mm, whilst frank invasion is recognised in widely invasive CXPA [10, 35, 83].
A rather extensive review indicates that poorly differentiated carcinoma and salivary duct carcinoma to be by far the two most common histological types of CXPA [32]. In the study of 73 cases of CXPA by Lewis and associates [49], there were 31 adenocarcinoma NOS, 24 salivary duct carcinoma, 5 adenosquamous carcinoma, 3 adenoid cystic carcinoma, 2 myoepithelial carcinoma and one each of epithelial-myoepithelial carcinoma and sarcomatoid carcinoma. Four of the 73 cases were intracapsular CXPAs. In a smaller series of 16 cases of CXPA, only 75 % of cases were purely epithelial and the remaining 25 % were carcinomas with a myoepithelial component [1]. The concept of a non-invasive (intracapsular, in situ) CXPA was introduced in 1977 by LiVolsi and Perzin [51], although ‘mixed tumours with suspicious areas’ were described earlier by Patey and associates in 1965 [63]. In the study by LiVolsi and Perzin, 6 of 47 cases of CXPA had foci of non-invasive adenocarcinoma in otherwise benign pleomorphic adenoma. These lesions were well circumscribed or encapsulated and showed marked cellular anaplasia and interpreted as in situ carcinoma [51]. The concept of non-invasive, in situ carcinoma ex pleomorphic adenoma is today well recognised hence show foci with carcinoma intermingled with the pleomorphic adenoma. Sometimes the inner ductal layers of the pleomorphic adenoma are seen to be replaced by carcinoma cells leaving surrounding outer benign appearing myoepithelial cells. The luminal (ductal) cells show a significant nuclear polymorphism often with prominent nucleoli and are enveloped by myoepithelial cells. If the atypia is less pronounced and small amounts of glands are involved, the differential diagnosis of pleomorphic adenoma with atypical histological features may be difficult (see Chap. 3). Likely the surrounding myoepithelial cells are also neoplastic in most instances which would explain the development not only of pure adenocarcinomas but also other malignancies with myoepithelial participation, e.g. adenoid cystic carcinoma, myoepithelial carcinoma, epithelial-myoepithelial carcinoma, etc. The pleomorphic adenoma component may be very small, frequently and extensively hyalinised, and may therefore be overlooked. Further, the malignant component is often a poorly differentiated carcinoma not readily recognised as salivary gland malignancy. These histological features may in cases with no clinical information about previous pleomorphic adenoma available at the time of examining the slides hinder reaching the correct diagnosis. The true nature of non-invasive CXPA has been debated in the past with arguments that the ‘carcinomatous’ change is not genuine but represents metaplastic changes into bizarre cells in a pleomorphic adenoma. The study by Di Palma and co-workers [22] clearly documented amplification of HER2 gene signals in tumour cells four out six tumours as studied by FISH, which strongly support that the cells are part of a focal non-invasive carcinoma and not reactive or metaplastic, bizarre cells. The authors also recommend the use of immunohistochemical staining for Ki-67 and HER2 as a conjunctive tool. A high Ki-67 index and a positive membranous HER staining will help in many cases, particularly when the malignant component consists of a salivary duct carcinoma, but not all salivary carcinomas stain positive for HER2. In a series of 19 cases of CXPA (conventional invasive CXPA), only 4 (21.1 %) showed membranous c-erbB-2 reactivity [70] and only 4 out 10 (40 %) in another study [56] (see below; Sect. 10.3.1). The biological behaviour of intracapsular (non-invasive) carcinoma ex pleomorphic adenoma is generally regarded as not aggressive [21], but the odd case with lung metastasis has been described [87]. This latter case report had histologically benign metastasis to the lung which thus gives further support for a continuum between metastasising pleomorphic adenoma and carcinoma ex pleomorphic adenoma [87] (see also Chaps. 3 and 15).
The histological distinction between non-invasive and minimal invasive CXPA lies in identifying the invasion through the capsule. Benign pleomorphic adenoma often has pushing margins and also frequently capsule violation, particularly in areas with a thin capsule. This should not be misinterpreted as invasion outside the capsule. The measurement is done in a perpendicular plane from the outer limit of the fibrous capsule with an ocular micrometre. Still, there may be cases when the extent of invasion cannot definitely be established, particularly so in minor salivary CXPA where the capsule rarely is well defined. The invasive tumour usually consists of small nests of tumour cells or neoplastic glands. The infiltrative areas are often irregularly shaped but also discontinuous satellite tumour nodules as well as broader pushing invasion can be seen. In minimally invasive CXPA, the invasion is limited to ≤1.5 mm (Fig. 10.2).
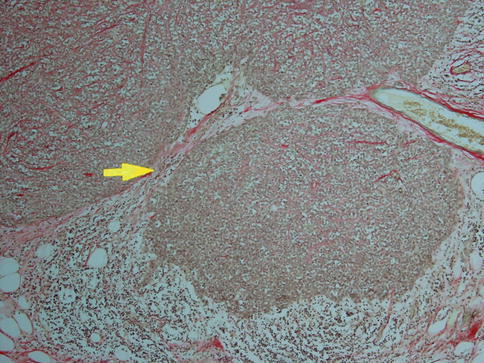

Fig. 10.2
Minimally invasive CXPA. Main tumour right being surrounded by a thin capsule (arrow) and invasion outside the capsule to a depth of 1.4 mm
The diagnosis of widely invasive CXPA rarely poses any problems. In almost half of cases, the tumour is a poorly differentiated adenocarcinoma that cannot readily be classified as any of the recognisable types of salivary adenocarcinomas. Should a history of previous pleomorphic adenoma not be known at the time of microscopic examination, or too few samples taken and no areas with pleomorphic adenoma present, this can be misinterpreted as metastatic adenocarcinoma (Fig. 10.3). In the majority of the other cases, however, the features are partly or entirely those of salivary duct carcinoma (see Chap. 11). Positive immunostainings for HER2/neu and androgen receptor will support the diagnosis of salivary duct carcinoma (Fig. 10.4). Myoepithelial carcinoma probably constitutes the third most common carcinoma component in CXPA (Fig. 10.5a). Even though myoepithelial carcinoma still relatively rarely constitutes the malignant component in CXPA, it more often arises in association with a pleomorphic adenoma than it does de novo [72]. Myoepithelial carcinoma may also be the metastatic component from CXPA even when the original malignant component of the CXPA has not been a myoepithelial carcinoma. A salivary duct carcinoma originated in a parotid pleomorphic adenoma in a 65-year-old man and 1 year later he developed bone metastasis of myoepithelial carcinoma (Fig. 10.5b). Carcinosarcoma (true malignant mixed tumour) is a very rare salivary malignancy and approximately 25 % of the 40–50 cases reported in the literature developed in a pre-existing pleomorphic adenoma (see Chap. 14). Carcinosarcoma is a biphasic tumour with varying proportions and types of carcinomatous and sarcomatous elements. Sarcoma is usually the principal tissue and chondrosarcoma, the most common type of sarcoma (Fig. 10.6).
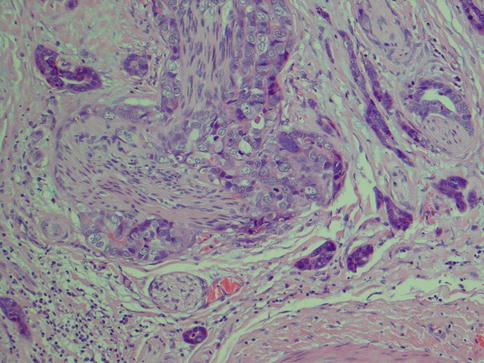
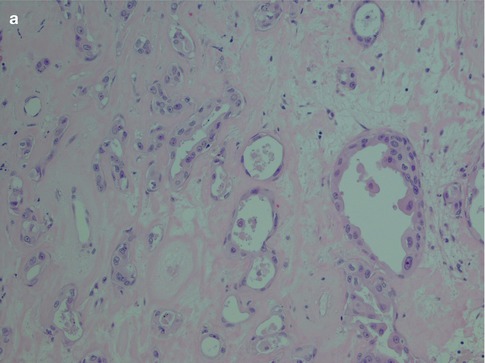
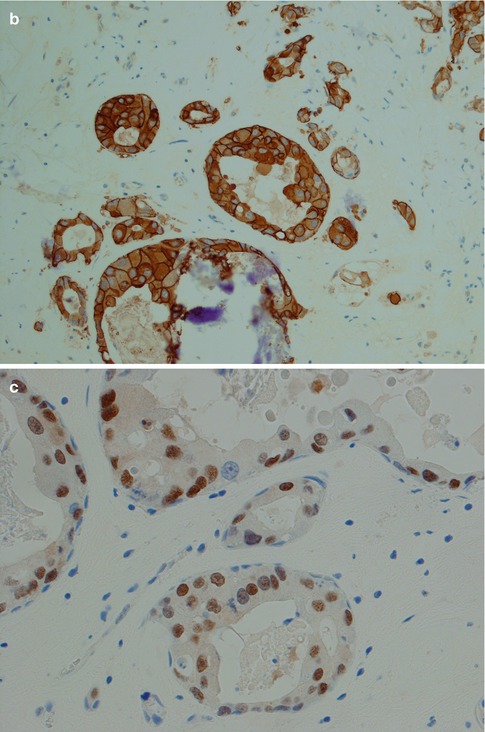
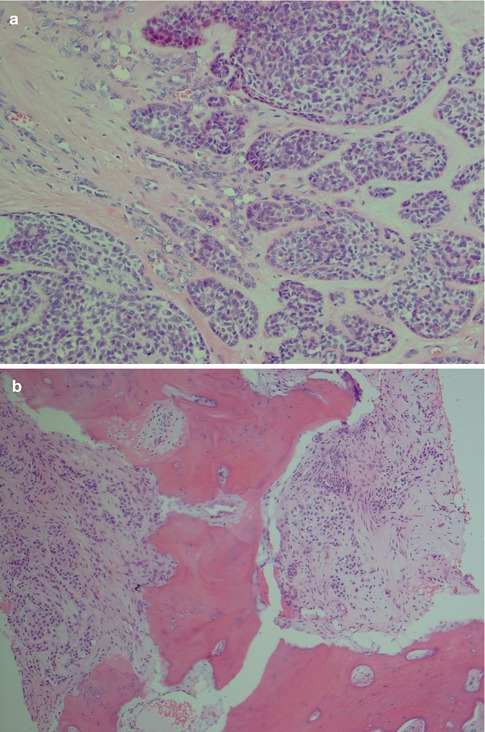
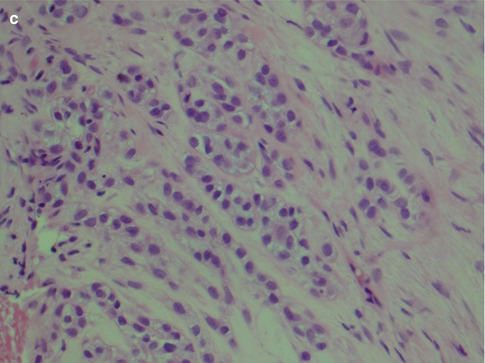
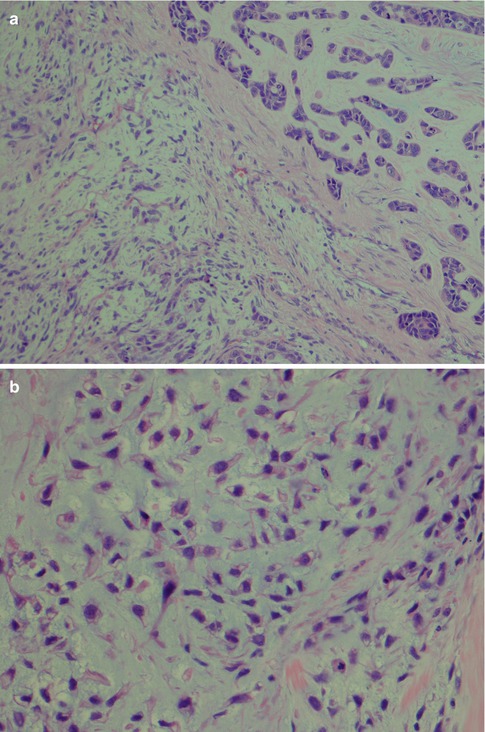

Fig. 10.3
CXPA consisting of a poorly differentiated adenocarcinoma. There are no distinguishing features of any distinct salivary carcinoma entity. Based on clinical history of recurrent PA, and clearly identified remnants of PA areas of the tumour, metastatic carcinoma was excluded


Fig. 10.4
(a) CXPA with features of salivary duct carcinoma. (b) Strong HER2 staining. (c) This tumour was also positive for androgen receptor


Fig. 10.5
(a) Myoepithelial carcinoma arising 13 years after resection of a pleomorphic adenoma. The tumour cells invade as single cells as well as in small nodular nests. (b) Metastatic myoepithelial carcinoma in the bone (L5) from a parotid CXPA. (c) Higher magnification showing bland epithelioid and clear myoepithelial cells

Fig. 10.6
Parotid CXPA with a carcinosarcoma as the malignant component. (a) Sarcoma (left) adjacent to a ductal carcinoma (right). (b) Higher magnification of the myxoid chondrosarcoma
10.3.1 Immunohistochemistry
Carcinoma ex pleomorphic adenoma has the translocations/rearrangements resulting in gene fusions involving the transcription factor genes PLAG1 and HMGA2 [5, 6, 31, 43, 64]. Also a t(3;8)(p21;q13) translocation has been detected as the sole chromosomal abnormality in a case of myoepithelial carcinoma ex pleomorphic adenoma [8] (for further reading, see Sect. 16.1).
The carcinoma and the benign component may show different immunoprofiles and that of the carcinoma can be reflected by the histological type. Pancytokeratins, AE1/AE3, CK7, CK8 and CK19 are positive in a very high proportion of cases but that is of little diagnostic help being positive in most salivary carcinomas. CK14 is preferentially expressed in ductal rather than myoepithelial elements of pleomorphic adenoma, but the reverse appears to be the case in CXPA [1, 18]. Expression of c-erbB-2/HER2/neu is not as common as previously may have been thought. It is present in a very high proportion of CXPA when there is an element of salivary duct carcinoma [22], but the overall positivity in CXPA (regardless carcinoma entity) does not appear to be more than 30–35 % of cases [49, 56, 70]. A recent study by Hashimoto and co-workers investigated HER2 overexpression and HER2 amplification in a series of 31 cases of CXPA with ductal differentiation (8 intraductal, 5 intracapsular and 18 extracapsular), and 7 cases of atypical pleomorphic adenomas (see Chap. 3). Their results suggest that HER2 may play a role in the progression of CXPA as HER2 amplification was more prevalent in extracapsular CXPAs than in intracapsular CXPAs [36]. EMA and CEA are positive in many cases (85 and 75 %, respectively), and CK20 is not always negative [49]. Cyclin A has recently been shown to have a significantly higher expression in the epithelial component of CXPA than in benign pleomorphic adenoma. Cyclin A is not expressed in normal salivary gland tissue [61]. Similarly, cyclin D1 is significantly more expressed in CXPA than in pleomorphic adenoma, indicating a possible role for cyclin D1 in the progression of pleomorphic adenoma to CXPA [62]. Maspin, the protease inhibitor that likely acts by inhibiting tumour cell invasion and motility, is strongly expressed in benign pleomorphic adenomas but virtually absent in the purely epithelial component of CXPA. The neoplastic myoepithelial cells of CXPA appear however to retain the maspin immunoreactivity [55]. The role of androgen receptors in CXPA remains unclear [58, 79]. The expressions of p16 and p21 are altered between PA and CXPA [78, 80, 81], and both p16 and p21 may have a role in the development of CXPA but are so far of no practical value in the diagnostic work.
10.4 Treatment and Prognosis
The risk of malignant transformation is generally considered to be greater the longer the tumour has been present and also increases with the number of recurrences. This time-dependent risk of malignant transformation has been estimated to 1.6 % in cases of pleomorphic adenomas being present for less than 5 years and rising to 9.6 % for those present for more than 15 years [23, 25]. Carcinomas ex pleomorphic adenomas are usually high-grade carcinomas, with frequent metastases and a poor clinical outcome. Radical surgery with neck dissection and also adjuvant radiation therapy is recommended for invasive CXPA, whilst radical surgery and follow-up are regarded as adequate for intracapsular and minimally invasive CXPA [2, 23, 32, 34, 35, 49, 51]. Non-invasive and minimally invasive CXPA appear to carry an excellent prognosis although frank malignancy may develop in rare cases. These subtypes of CXPA have not been widely recognised for that many years and further studies are therefore needed to fully evaluate their clinical behaviour. They can have unusual clinical presentations as a recently reported case of non-invasive CXPA (adenocarcinoma) where after 8 years the benign pleomorphic adenoma component recurred. After yet another 5 years, the tumour recurred again but this time as a myoepithelial carcinoma [52]. Cases of intracapsular CXPA with regional lymph node metastases, and with distant lung metastasis, have been reported, emphasising that although these subtypes of CXPA have a very benign clinical behaviour, they are not completely devoid of metastatic potential [26, 87]. We have very recently come across yet another case of non-invasive CXPA where distant spinal metastases have developed 1 year after parotidectomy. It may be prudent to keep the patients with the non-invasive and minimal invasive types of CXPA under observation until more data is available to really confirm that these tumours do have an excellence prognosis or not. A relatively recent study on this theme suggested that the threshold for distinguishing minor extracapsular invasion with good prognosis from wide extracapsular invasion with poor prognosis is 5 mm [86]. Histological subtype of the carcinoma component appears to play a prognostic role, particularly in cases of intracapsular/minimally invasive CXPAs, where the presence of myoepithelial carcinoma carries a worse prognosis [44].
CD34 is a panendothelial marker and stains newly formed vessels but also old ones trapped in and around the tumours. CD105 (endoglin), on the other hand, is considered a marker of neoangiogenesis. Staining with CD105 in a series of pleomorphic adenoma and carcinoma ex pleomorphic adenomas has suggested that there is an angiogenic switch during the progression from adenoma to carcinomas [76]. As is the case with pleomorphic adenoma, CXPA often shows alterations of chromosome 8q12 and 12q13-15. Rearrangements of 8q12 are a frequent finding but also alterations at 12q13-15. Studies by El-Naggar and associates indicate that there is a higher incidence of loss of heterozygosity at chromosome arms 8q and 12q than at 17p loci in pleomorphic adenomas and in the adenoma component of CXPA. The carcinoma component in CXPA also had combined LOH at these three chromosome arms but a higher incidence, particularly at 17p. The chromosome arm 17p alterations also correlated significantly with high disease stage. The authors conclude that alterations at 8q and/or 12q may constitute early events associated with pleomorphic adenomas and that LOH at 12q loci may identify a subset of adenoma with potential progression to carcinoma. Further acquisition of additional alterations at 17p loci may represent events preceding malignant transformation and progression [24]. Target genes creating fusion oncogenes like, e.g. PLAG1-CTNNB1 in pleomorphic adenoma and MECT1-MAML2 in mucoepidermoid carcinoma have been recognised (see Chaps. 3 and 7). The WNT inhibitory factor 1 (WIF1) gene has been reported as a novel HMGA2 (high mobility group protein gene) fusion partner in pleomorphic adenoma. WIF1 rearrangement was also detected in one case of CXPA. The rearrangement of one WIF1 allele coexisted with the loss of the other allele, a typical signature of a tumour suppressor gene. WIF1 is an antagonist of the Wnt signalling pathway, and the findings suggest that downregulation of WIF1 may play a role in the development and/or progression of pleomorphic adenomas [67].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


