chapter 8 Carbohydrates: strong and sweet
Carbohydrates or sugars, as they are often called, are the most abundant organic molecules found in living organisms. Indeed, more than half of the organic carbon on Earth is stored as two carbohydrate molecules, namely starch and cellulose. Carbohydrates play a wide variety of biological roles such as providing dietary energy for many organisms, acting as an energy storage depot for plants and animals, playing a role in cellular communication, and carrying out many structural roles in cells. The name for these molecules is derived from ‘hydrates of carbon’ and comes from elemental analysis of simple carbohydrates which gives a general formula of Cn(H2O)n.
Classification of carbohydrates
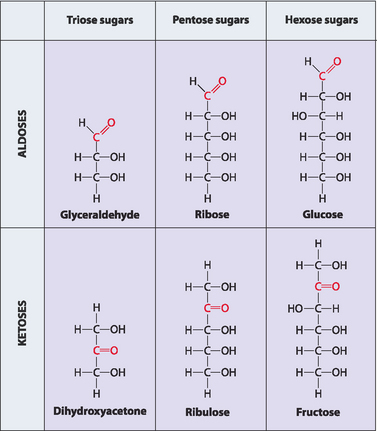
Simple sugars, or monosaccharides, are initially classified by the number of carbon atoms they contain (Table 8-1), and are then divided into two broad families depending on whether their most oxidised functional group is an aldehyde (aldose sugars) or a ketone (ketose sugars) (Fig 8-1). The suffix ‘ose’ indicates that a free carbonyl group is present. Monosaccharides can be linked together using a special type of chemical bond known as a glycosidic bond (see below) in a manner analogous to amino acids being joined by peptide bonds to make polypeptides. The names of these larger assemblages are based on the number of monomers used to make them. When two monosaccharides are joined the resulting molecules are referred to as disaccharides, three to twelve joined together give oligosaccharides, and more than twelve give polysaccharides.
TABLE 8-1 Examples of important monosaccharides found in humans
| Number of carbon atoms | Generic name | Example |
|---|---|---|
| 3 | Triose | Glyceraldehyde |
| 5 | Pentose | Ribose |
| 6 | Hexose | Glucose |
Isomeric forms
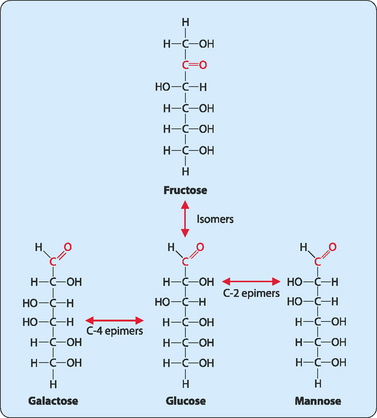
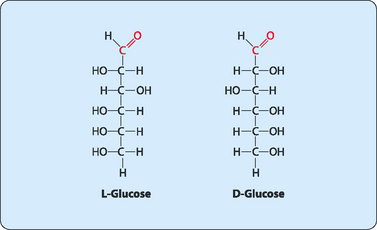
Many sugars have the same chemical formula but different structures. For example fructose, glucose, mannose and galactose all have different structures but the same chemical formula (C6H12O6), and are therefore isomers of each other (Fig 8-2). If the structures of the isomers vary only at a single carbon (not including the carbonyl carbon) atom then they are termed epimers of each other. Molecules that have structures which are mirror images of each other display a special form of isomerism and are referred to as enantiomers (Fig 8-3). Each member of the pair is defined as the D- or L-form, with most sugars in humans being in the D-form.
Linear or cyclic?
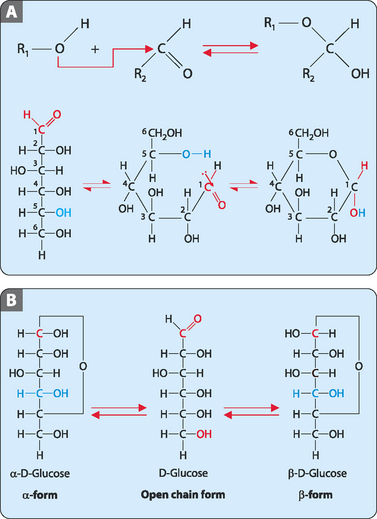
Most sugars with five or six carbon atoms exist predominantly as cyclic molecules rather than as the linear open-chain forms. The cyclic molecules are generated by the interaction of the carbonyl group of the aldehyde or ketone with a distant functional group to give a hemiacetal in aldoses or a hemiketal in ketoses (Fig 8-4A). Hemiacetals and hemiketals are derived from the addition of an aldehyde or ketone carbonyl group to an alcohol. The general formula of a hemiacetal is R1R2C(OH)OR, where R1 or R2 is often hydrogen and R (bonded to O) is not hydrogen. In a hemiketal none of the R-groups can be a hydrogen atom. The carbonyl carbon then becomes a new chiral centre and is referred to as the anomeric carbon. The cyclic molecules formed can exist in two forms, which are termed anomers, and are designated α and β (Fig 8-4B), with the α-isomer having the hydroxyl group attached to the anomeric carbon below the plane of the carbon atoms, and the β-isomer having the hydroxyl of the anomeric carbon above the plane.