Chapter 20 Carbohydrates
As the name implies, carbohydrates consist of carbon, hydrogen and oxygen with the last two elements usually present in the same proportions as in water. As we have previously noted, carbohydrates are among the first products to arise as a result of photosynthesis. They constitute a large proportion of the plant biomass and are responsible, as cellulose, for the rigid cellular framework and, as starch, for providing an important food reserve. Of special pharmacognostical importance is the fact that sugars unite with a wide variety of other compounds to form glycosides (Chapters 21–25). Mucilages, as found in marshmallow root and psyllium seeds, act as water-retaining vehicles, whereas gums, which are similar in composition and properties, are formed in the plant by injury or stress and usually appear as solidified exudates; both are typically composed of uronic acid and sugar units. The cell walls of the brown seaweeds and the middle lamellae of higher plant tissues contain polysaccharides consisting almost entirely of uronic acid components. All these groups are discussed more fully below, and the drugs and pharmaceutical necessities containing them are listed at the end of the chapter.
SUGARS (SACCHARIDES)
Monosaccharides
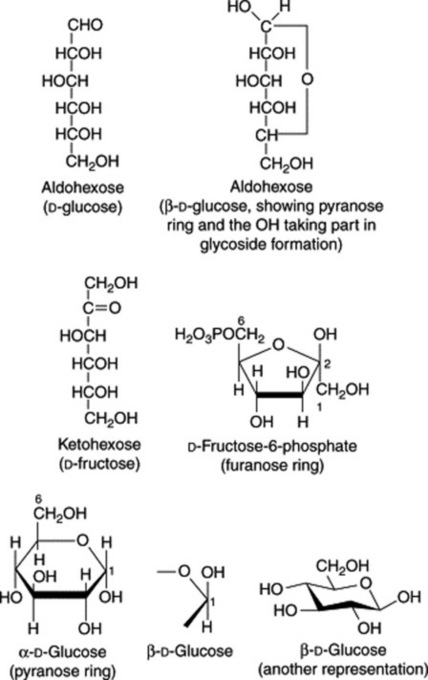
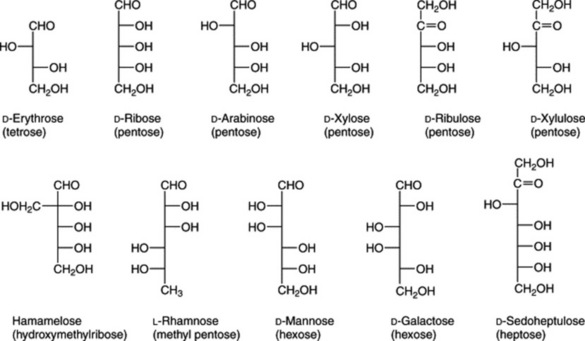
The formulae of sugars and other carbohydrates are written in a number of different ways. The structure of glucose as a straight-chain pentahydroxy aldehyde was established by Kiliani in 1886. Emil Fischer, from 1884 onwards, was the most important of the early workers in this field. Their straight-chain formulae are still useful for illustrating the isomerism and stereochemical relationships and, as shown below, can be written in very abbreviated form. Many of the important biological properties of carbohydrates can, however, best be illustrated by ring formulae which show that the same sugar may exist either as a five-membered ring (furanose) or a six-membered ring (pyranose). Glucose has an aldehyde group and is therefore called an aldose or ‘aldo’ sugar; fructose has a ketone group and is therefore called a ketose. Terms such as ‘aldopentose’ and ‘ketohexose’ are self-explanatory. The formulae (Figs. 20.1, 20.2) illustrate these points.
The furanose structure is comparatively unstable but may be stabilized on glycoside formation. The fructose phosphate of the furanose form illustrated in Fig. 20.1 is an intermediate in glycolysis, the anaerobic degradation of hexoses which provides energy for metabolism (see Fig. 18.5). Fructose in nature is always in the furanose form, but when isolated in crystalline form, it has a pyranose structure.
Di-, tri- and tetrasaccharides
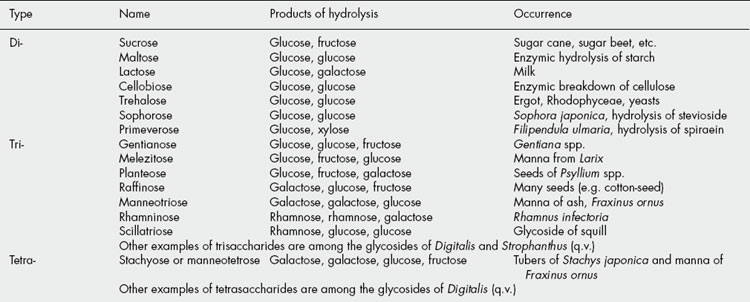
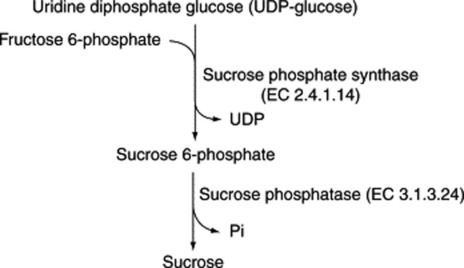
These sugars may also be called bioses, trioses and tetroses. They are theoretically derived from two, three or four monosaccharide molecules, respectively, with the elimination of one, two or three molecules of water (Table 20.1). One of the commonest plant disaccharides is sucrose; it is formed in photosynthesis by the reaction of UDPG with fructose-6-phosphate (Fig. 20.3). Control mechanisms for the build-up of sucrose in leaves, and its breakdown for transport to storage organs, are achieved by metabolite effector control of the appropriate enzymes.
POLYSACCHARIDES
Table 20.2 indicates the character of some of the polysaccharides.
Table 20.2 The character of some polysaccharides.
| Name | Occurrence and nature |
|---|---|
| Containing only monosaccharide units | |
| 1. Amylopectin or α-amylose | The main constituent of most starches (over 80%). The molecule has branched chains each consisting of 20–26 α-1,4-linked glucose residues. Several hundred of these chains are linked by α-1,6 glycosidic bonds to neighbouring chains giving a molecule containing some 50 000 glycosyl units. The branching pattern throughout the molecule is not uniform, resulting in some areas that are apparently amorphous (high degree of branching) and others probably crystalline (linear chains predominate with little branching) |
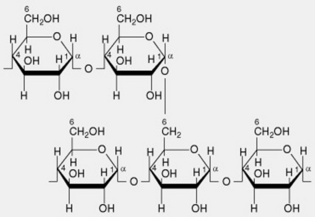 | |
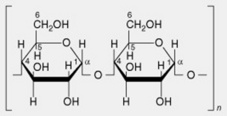
| 2. Amylose or β-amylose | Most starches contain up to 20%, but sometimes absent. Consists essentially of linear chains of α-1,4-linked glucose residues. Several thousand glucose units constitute a chain. It is now recognized that there is a very limited branching (α-1,6-linkages) to the extent of 2–8 branches per molecule |
 | |
| 3. Glycogen or animal starch | Important reserve carbohydrate of animal tissues. Molecule resembles that of amylopectin |
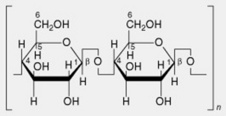
| 4. Cellulose | Chief polysaccharide of plant cell walls. Linear chains of β-1,4-linked glucose residues |
 | |
| 5. Inulin | A reserve carbohydrate particularly abundant in the Compositae. Linear chains of up to 50 β-1,2-linked fructofuranose units terminated by a single glucose unit |
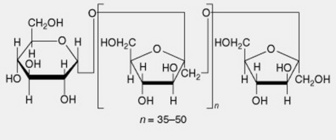 | |
| 6. Xylans, mannans and galactans | These are often associated with one another and with cellulose. They are difficult to isolate in a pure form. On hydrolysis they yield xylose, mannose and galactose, respectively |
| 7. Hemicelluloses | These polysaccharides occur in the cell wall with cellulose and pectic substances. The nomenclature, dating from 1891, is deceptive because hemicelluloses are not components of cellulose but are formed mainly from hexose and pentose units. Hemicelluloses vary according to source and can be classified as xylans, mannans and galactans according to their principal components |
| 8. Lichenin or lichen starch | A polysaccharide found in lichens. Resembles cellulose but molecule contains about 25% of β-1,3 glucosidic linkages |
| Polysaccharide complexes containing uronic acid or other units | |
| 1. Pectins | These occur in the middle lamellae of cell walls and are abundant in fruits (e.g. apples, oranges) and roots (beets and gentian). The parent substance protopectin is insoluble but is easily converted by restricted hydrolysis into pectinic acids (pectins). Pectins from different sources vary in their complex constitution, the principal components being blocks of D-galacturonic acid residues linked by α-1,4- glycosidic linkages and interspersed with rhamnose units; some of the carboxyl groups are methylated. These molecules are accompanied by small amounts of neutral arabinans (branched polymers of α-1,5-linked L-arabofuranose units) and galactans (largely linear chains of β-1,4- linked D-galactopyranose units) |
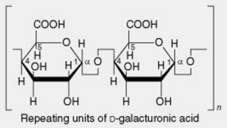 | |
| 2. Algin or alginic acid | Alginic acid is the principal constituent of the cell walls of the brown algae. It was discovered by Stanford in 1880 and is now widely used for the manufacture of alginate salts and fibres (q.v.). The composition varies according to the biological source, thus providing a range of properties which are exploited commercially. It is a heteropolyuronide consisting of chains of β-1,4-linked D-mannuronic acid units interspersed with lengths of α-1,4-linked L-guluronic acid units together with sections in which the two monouronide units are regularly interspersed. In alginic acids from different sources the ratios of the two uronic acids vary from 2:1 to 1:2. The chain length varies with the method of preparation and molecular weight, and viscosity measurements suggest molecules of from 220 to 860 units |
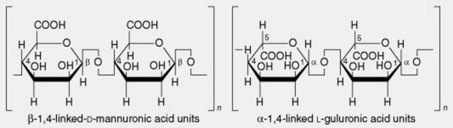 | |
| 3. Polysaccharides with sulphuric acid esters | Certain algae, including those yielding agar and carrageen, contain a mixture of polysaccharides. Agar, sulphuric acid esters for example, contains a biose formed from D– and L-galactose but also a more complex agaropectin formed from galactose and uronic acid units partly esterified with sulphuric acid. Carrageen has a similar composition |
| 4. Chitin | This is found in some of the lower plants, in insects and in crustaceans. The molecule consists of linear chains of β-1,4-linked N-acetyl-D-glycosamine residues. Its inclusion in the microfibrillar component of the fungal cell wall is analogous to that of the cellulose microfibril |
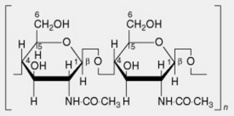 | |
| 5. Gums and mucilages | Gums such as acacia and tragacanth and mucilages, such as those found in linseed, psyllium seeds and marshmallow root, are found in many plants, where they are usually formed from the cell wall or deposited on it in layers. They are essentially polyuronides consisting of sugar and uronic acid units. Some gums have methoxyl groups (e.g. tragacanth); in others the acidic complex is united with metals (e.g. acacia) |
In addition to the well-established polysaccharide-containing pharmaceutical materials described later in this chapter there is now considerable interest in a number of polysaccharides with other pharmacological activities. These include immuno-modulating, antitumour, anti-inflammatory, anticoagulant, hypoglycaemic and antiviral properties. Specific examples are the glycyrrhizans of Glycyrrhiza uralensis and G. glabra and the glycans of ginseng and Eleutherococcus (q.v.). In general polysaccharides from fungi exhibit antitumour activity, those from higher plants are immunostimulatory and the algal polysaccharides, which often contain sulphate, are good anticoagulants.
Tests for carbohydrates
The following are some of the more useful tests for sugars and other carbohydrates.
COMMERCIAL PLANT-DERIVED FIBRES AND PRODUCTS
The biological origin and the structure of plant fibres is discussed in Chapter 42; many have important commercial uses and for a review on their botany, chemistry and processing see McDougall et al., J. Sci. Food, Agric., 1993, 62, 1.
COTTON, RAW COTTON
Microscopy of unbleached cotton
Cotton consists of unicellular hairs the appearance of which has been likened to that of empty, twisted fire-hoses. Their length is up to about 5 cm, diameter 9–24 μm, and the number of twists varies from about 75 cm−1 in the Indian to 150 cm−1 in the Sea Island. Pieces of ‘shell’ or seed coat, which can often be picked from samples of raw cotton, show hair bases fitting between the thick-walled epidermal cells. The apex is rounded and solid. The cotton hair is cylindrical when young but becomes flattened and twisted as it matures, the large lumen, which contains the remains of protoplasm being much elongated in transverse section. The cellulose wall of the hair is covered with a waxy cuticle which renders it non-absorbent. The cuticle may be stained with ruthenium red. Bleached cotton yarn and absorbent cotton wool (see below) are readily wetted by water.
Tests
The following tests are applicable to cotton.
ABSORBENT COTTON WOOL, ABSORBENT WOOL
Chemical nature
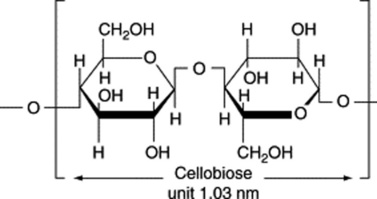
Absorbent cotton is a very pure form of cellulose and its chemical and physical properties have been extensively studied. The cellulose molecule is built up of glucose residues united by 1,4-β-glucosidic links (contrast starch). The wall of the cotton fibre, like that of plant cells in general, shows anisotropic properties. When swollen in water, the swelling is in a direction at right angles to the long axis. In the direction of the long axis it shows considerable tensile strength. Examined in polarized light, it shows birefringence, the value of the double refraction depending on the liquid in which the fibre is immersed. This phenomenon, characteristic of mixed bodies with rod-like structural elements, has been termed rodlet-double refraction. Stained with chlor-zinc-iodine and examined microscopically in polarized light (analyser removed), the fibre shows greater absorption when orientated with its long axis parallel to the plane of polarization than when orientated with the long axis at right angles (dichroism). These physical properties suggest that the fibre wall is built up of elongated structural units orientated in some definite manner. The study of the cotton fibre by X-ray analysis has confirmed this and has shown that its cell wall is composed of elongated chain-like molecules (built up of repeating units 1.03 nm long) and orientated in a spiral manner, the spiral making an angle of 30° with the long axis of the fibre. The length of the repeating unit of structure corresponds to that of two glucose residues fully extended. This unit is the ‘cellobiose unit’, many of which are united in the polysaccharide molecule of cellulose (Table 20.2).
The biosynthesis of cellulose in the cotton trichome would appear to involve UDP-glucose originating from sucrose.
REGENERATED CARBOHYDRATE MATERIAL AND CHEMICALLY MODIFIED FIBRES
Viscose (regenerated cellulose, rayon)
Viscose rayon is a very pure form of cellulose. It yields a trace of ash which contains sulphur. The cellulose molecules of the original natural material, whether wood or cotton, become more separated from one another in the viscose solution than in the vegetable material and in the regenerated fibre are still less closely packed. Radiography has shown that the side-to-side aggregation of the long-chain molecules is different from that in natural celluloses. The size of the molecules is also reduced, wood cellulose having molecules of the order of 9000 glucose residue units, while those of viscose rayon have only about 450.
Chemical tests
Oxidized cellulose
Oxidized cellulose originated in the USA as a result of the work published by Yackel and Kenyon in 1942. Cotton wool or gauze is treated with nitrogen dioxide until the number of carboxyl groups formed bythe oxidation of the primary alcohol groups of the glucose residue units of the cellulose molecules reaches 16–22%. The original cellulose now has glucuronic acid residue units (compare alginic acid) as well as some glucose residue units.
Tests
Alginate fibres
These originated about 1938 in Britain and were further developed during World War II.
As indicated in Table 20.2, alginic acid is composed of polymers of both mannuronic and guluronic acids. The properties of the two are variable and alginates of different origin have different compositions and properties. This is illustrated by the two commercial haemostatic dressings—Kalostat (BritCair Limited) and Sorbsan (Steriseal—Pharmaplast Limited). The former is derived from the seaweed Laminaria hyperborea collected off the Norwegian coast and yields an alginate with a guluronic:mannuronic ratio of 2:1; the latter is prepared from Laminaria and Ascophyllum species collected off the west coast of Scotland and gives an alginate with a guluronic:mannuronic acid ratio of about 1:2. On a wound surface the α-linkages of the guluronic acid polymer are not easily broken so that fibre strength is retained and a strong gel is formed on contact with the wound exudate. A high ratio of mannuronic acid polymer (β-linkages) yields a product giving a weaker gel and less retention of fibre strength. In practice this means that the Kalostat dressing can be removed from the wound with forceps and Sorbsan is removed by irrigation with, for example, sodium citrate solution.
Alginate filaments are composed of salts of the long-chain molecules of alginic acid (see Table 20.2) and there is little cross-linking between the chains in the fibre.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree