Objectives
- Understand the barriers to assimilation of water-soluble macromolecules into the body
- Describe dietary sources of carbohydrates, and the pathways involved in the digestion and absorption of carbohydrate polymers, dietary disaccharides, and monosaccharides
- Define the relative roles of luminal and brush border digestion for each type of carbohydrate
- Identify the membrane transporters involved in the uptake of monosaccharides
- Define the relative roles of luminal and brush border digestion for each type of carbohydrate
- Describe how carbohydrate assimilation is regulated during development and by specific dietary components
- Compare and contrast protein assimilation with that of carbohydrates
- Identify essential amino acids, and understand why they must be provided in the diet
- Describe pathways involved in the digestion and absorption of proteins, peptides, and amino acids
- Define the relative roles of luminal, brush border, and cytosolic digestion for each substrate
- Explain how the epithelium can transport a diverse set of peptides into the body
- Define the relative roles of luminal, brush border, and cytosolic digestion for each substrate
- Understand how protein assimilation is regulated
- Define how key water-soluble vitamins are taken up into the body
- Describe conditions where the absorption of water-soluble components of the diet is abnormal
- Appreciate the basis of lactose intolerance, and why it is common in adults
Basic Principles of Carbohydrate and Protein Assimilation
It is perhaps ironic that it is only now, at the end of this volume, that we come to discuss in detail the processes that underpin what is arguably the most important physiologic function of the gastrointestinal system—namely, the assimilation of nutrients into the body. However, the author hopes that, by having provided thorough discussions of the secretory and motor functions of the gut, students will now be in a position to rapidly appreciate how these functions are ultimately integrated to respond to the ingestion of a meal.
 Carbohydrates and proteins are water-soluble macromolecules of nutritional significance. Together with lipids, which will be discussed in the next chapter, they represent the major sources of calories in the diet, and each supplies specific building blocks for molecules needed for the physiologic function of the body as a whole. Dietary carbohydrates are the major exogenous source of glucose, which is utilized by cells as an energy source. Nutritionally significant carbohydrates include both large polymers and disaccharides consisting of two sugar molecules bound together (Table 15–1). Proteins supply amino acids, which are resynthesized into new proteins needed by the body. While the body can synthesize glucose de novo from a variety of substrates, as described earlier, some amino acids cannot be synthesized by the body. These are the so-called essential amino acids, which will be described in more detail later in this chapter.
Carbohydrates and proteins are water-soluble macromolecules of nutritional significance. Together with lipids, which will be discussed in the next chapter, they represent the major sources of calories in the diet, and each supplies specific building blocks for molecules needed for the physiologic function of the body as a whole. Dietary carbohydrates are the major exogenous source of glucose, which is utilized by cells as an energy source. Nutritionally significant carbohydrates include both large polymers and disaccharides consisting of two sugar molecules bound together (Table 15–1). Proteins supply amino acids, which are resynthesized into new proteins needed by the body. While the body can synthesize glucose de novo from a variety of substrates, as described earlier, some amino acids cannot be synthesized by the body. These are the so-called essential amino acids, which will be described in more detail later in this chapter.


 Due to their hydrophilicity, proteins and carbohydrates are “at home” in the aqueous environment of the intestinal lumen. However, neither they, nor the water-soluble end products of their digestion, can readily traverse the membranes of the epithelial cells that line the small intestine. Moreover, intact dietary polymers are too large to be transported into cells. Thus, a series of ordered chemical reactions, catalyzed by specific hydrolase enzymes, break down both proteins and carbohydrate polymers to their component monomers or short oligomers thereof. Digestion of both carbohydrates and proteins takes place at two sites. First, enzymes secreted into the bulk contents of the intestinal lumen begin the digestive process. Second, membrane-bound hydrolases localized to the microvillous membrane (“brush border”) of the epithelial cells lining the villus tips in the small intestine mediate the next stage of digestion. The epithelium is only capable of transporting monosaccharides and so even dietary disaccharides must be digested at the brush border before they can be absorbed. For proteins, on the other hand, the epithelium expresses transporters that can take up short peptides, as well as those specific for monomeric amino acids. Thus, peptides taken up into the enterocytes undergo a third stage of digestion in the cytosol, mediated by intracellular hydrolases.
Due to their hydrophilicity, proteins and carbohydrates are “at home” in the aqueous environment of the intestinal lumen. However, neither they, nor the water-soluble end products of their digestion, can readily traverse the membranes of the epithelial cells that line the small intestine. Moreover, intact dietary polymers are too large to be transported into cells. Thus, a series of ordered chemical reactions, catalyzed by specific hydrolase enzymes, break down both proteins and carbohydrate polymers to their component monomers or short oligomers thereof. Digestion of both carbohydrates and proteins takes place at two sites. First, enzymes secreted into the bulk contents of the intestinal lumen begin the digestive process. Second, membrane-bound hydrolases localized to the microvillous membrane (“brush border”) of the epithelial cells lining the villus tips in the small intestine mediate the next stage of digestion. The epithelium is only capable of transporting monosaccharides and so even dietary disaccharides must be digested at the brush border before they can be absorbed. For proteins, on the other hand, the epithelium expresses transporters that can take up short peptides, as well as those specific for monomeric amino acids. Thus, peptides taken up into the enterocytes undergo a third stage of digestion in the cytosol, mediated by intracellular hydrolases.
Carbohydrate Assimilation
Even in the era of the Atkins diet and the like, where those wishing to lose weight adhere to a diet very low in these compounds, carbohydrates continue to represent a major component of the human diet (typically 40–60% of total calories), and assume particular importance in specific populations (Table 15–1). In developing countries, where protein is scarce, carbohydrates may be the major caloric source. There are three main forms of carbohydrate that have nutritional significance—starch, sucrose, and lactose. The proportion of calories obtained from each varies among different populations. For example, those in developing countries ingest most of their carbohydrate in the form of starch, whereas infants obtain the majority of their carbohydrate calories from lactose, which is found in breast milk. Finally, “Western” diets in industrialized countries tend to be rich in refined sugar (i.e., sucrose).
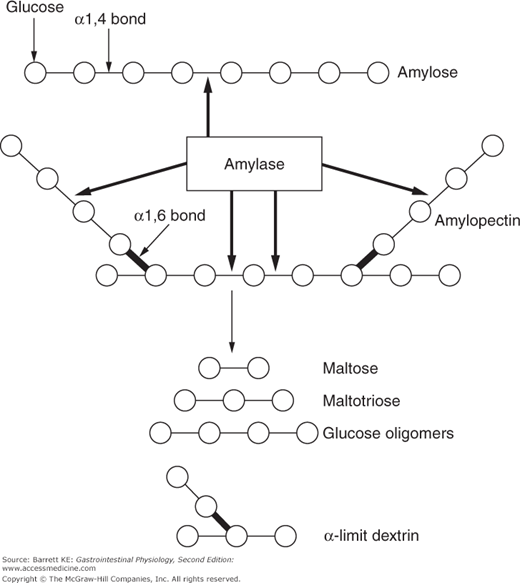
Starch is the name given to a complex mixture of dietary polymers of glucose, and is derived from a variety of plant sources. Starch is found in cereals, in breads, and in starchy vegetables such as potatoes. There are two different types of glucose polymers in starch, which is significant because they require different enzymes to digest them fully. About 25% of starch consists of amylose, which comprises simple, straight-chain polymers of glucose (Figure 15–1). The remainder of the nutritional portion of starch consists of amylopectin, which comprises complex, branched polymers of glucose (Figure 15–1).
Sources of starch also supply other carbohydrate polymers, as well as noncarbohydrate polymers, that collectively are known as dietary fiber. Fiber is characterized by the fact that its constituent polymers cannot be degraded by luminal hydrolases, including those secreted by the pancreas, nor by those expressed on the surface of the enterocytes. Fiber is critical for intestinal health because, being indigestible in the small intestine, it remains in the lumen and provides bulk to the stool, retaining fluid and aiding passage of the fecal material through the colon. This is the basis for so-called “bulk-forming” laxatives, which add additional fiber to the diet and can be used to relieve constipation. It also explains why those who consume a diet rich in fruits, vegetables, and unrefined grains rarely suffer from such problems. Fiber has additional nutritional significance in that, although it is not subject to digestion by mammalian enzymes, it can be broken down by hydrolases expressed by certain colonic bacteria. These reactions generate short-chain fatty acids, which are important energy sources for colonocytes (you will recall that we also discussed this topic in Chapter 6).
 Digestion of carbohydrates begins as soon as the meal is taken into the mouth. Saliva contains a 56-kDa amylase enzyme that is closely related to the 55-kDa amylase that is secreted into the pancreatic juice. As its name implies, salivary amylase is capable of digesting amylose, the straight-chain component of starch. Salivary amylase is not essential for the normal digestion of carbohydrates, since, as we have learned, all of the enzymes in the pancreatic juice are present in considerable excess of requirements. However, the salivary enzyme likely does assume an important role in specific situations. For example, in infants, there is a developmental delay in the production of pancreatic enzymes and so the salivary enzyme assumes a proportionately greater role. Salivary amylase is also an important backup in patients with pancreatic insufficiency, such as in the setting of cystic fibrosis.
Digestion of carbohydrates begins as soon as the meal is taken into the mouth. Saliva contains a 56-kDa amylase enzyme that is closely related to the 55-kDa amylase that is secreted into the pancreatic juice. As its name implies, salivary amylase is capable of digesting amylose, the straight-chain component of starch. Salivary amylase is not essential for the normal digestion of carbohydrates, since, as we have learned, all of the enzymes in the pancreatic juice are present in considerable excess of requirements. However, the salivary enzyme likely does assume an important role in specific situations. For example, in infants, there is a developmental delay in the production of pancreatic enzymes and so the salivary enzyme assumes a proportionately greater role. Salivary amylase is also an important backup in patients with pancreatic insufficiency, such as in the setting of cystic fibrosis.
Perhaps counterintuitively, salivary amylase is quite sensitive to acidic pH, and in theory its activity would be terminated as soon as the meal enters the stomach. However, it has been demonstrated that amylase activity can be protected if its substrate occupies the active site of the enzyme. Thus, while starch is present in the gastric lumen, it is likely that its digestion mediated by salivary amylase can continue, until the task is assumed by the pancreatic enzyme. The latter is also sensitive to acid, but acts in an environment where gastric juices have been neutralized by duodenal, pancreatic, and biliary bicarbonate secretion.
The synthesis and secretion of salivary amylase in the serous cells of the salivary glands are regulated by neurohumoral signals coincident with ingestion of a meal. Interestingly, in common with the pancreatic isoform, the synthesis of salivary amylase is upregulated by carbohydrate ingestion. Thus, the substrate controls the availability of the means of its digestion.
In health, the majority of starch digestion likely involves the 55-kDa amylase that is secreted as an active enzyme into the pancreatic juice by the pancreatic acinar cells (see Chapter 4). Both the pancreatic and salivary enzymes act rapidly to cleave starch into a mixture of products, depending on whether amylose or amylopectin is the substrate. The amylase enzymes target internal α-1,4 bonds of both molecules, but the terminal bonds, as well as the α-1,6 bonds that provide for the branched-chain structure of amylopectin, are resistant (Figure 15–1). This means that while the action of amylase is rapid, none of the products it generates can immediately be absorbed by the enterocytes, since we know that the epithelium can only transport monosaccharides. By the time the meal reaches the proximal small intestine, digestion of starch generates a mixture of maltose (a dimer of glucose), maltotriose (a trimer of glucose), and so-called α-limit dextrins, which are the simplest structures that can be derived from the branch points in amylopectin. Both maltose and maltotriose are resistant to amylase as they contain only terminal, and no internal, α-1,4 bonds.
Although the action of amylase is rapid, some sources of starch contain proteins and fiber components that may slow the action of this enzyme. This means that the rise in blood glucose that eventually follows the ingestion of starch will have differing kinetics depending on the food in which the starch is contained. Some nutritional supplement manufacturers have tried to take advantage of this by isolating so-called “starch blockers,” which they claim can reduce the digestion of starch by inhibiting amylase activity and thereby are useful as aids to weight loss. However, your knowledge of gastrointestinal physiology should tell you that this is unlikely to be an effective approach, given the marked excess of pancreatic enzymes and the fact that the starch blockers are proteins, and therefore are themselves digested by mechanisms as we will discuss for dietary proteins later in this chapter. Further, any carbohydrate that escapes assimilation in the small intestine is rapidly broken down by bacterial hydrolases in the colon. Although this may carry the price of the generation of gas and bloating, it ultimately allows much of the caloric content of the starch to be reclaimed.
 The products of luminal starch digestion, as well as dietary disaccharides, are then acted upon by specific hydrolases localized to the enterocyte brush borders. Brush border digestion is an essential component of the pathways leading to assimilation of all dietary carbohydrates, with the exception of glucose (which accounts for the inclusion of the latter in energy drinks, and the like, because it can therefore be rapidly absorbed). Brush border hydrolysis of carbohydrates, as well as other dietary components, likely increase the efficiency of carbohydrate absorption because the monosaccharides generated are produced in close proximity to the transporters that are then required for their uptake. Likewise, this may also sequester digested monosaccharides from the limited numbers of small intestinal bacteria that might otherwise be stimulated to proliferate by the availability of this source of nutrition.
The products of luminal starch digestion, as well as dietary disaccharides, are then acted upon by specific hydrolases localized to the enterocyte brush borders. Brush border digestion is an essential component of the pathways leading to assimilation of all dietary carbohydrates, with the exception of glucose (which accounts for the inclusion of the latter in energy drinks, and the like, because it can therefore be rapidly absorbed). Brush border hydrolysis of carbohydrates, as well as other dietary components, likely increase the efficiency of carbohydrate absorption because the monosaccharides generated are produced in close proximity to the transporters that are then required for their uptake. Likewise, this may also sequester digested monosaccharides from the limited numbers of small intestinal bacteria that might otherwise be stimulated to proliferate by the availability of this source of nutrition.
Brush border hydrolysis is catalyzed by a series of enzymes that are synthesized in the enterocytes as they differentiate along the crypt–villus axis. The enzymes are trafficked specifically to the apical membrane of these cells and anchored in the membrane by a single transmembrane segment. Brush border hydrolases are also heavily glycosylated as they are processed through the biosynthetic pathway. This may protect them, to some degree, from proteolysis catalyzed by the pancreatic proteases that are present in the lumen. The single polypeptide chains that constitute these hydrolases may be posttranslationally modified further to generate specific domains that may contain distinct or similar active sites. Finally, all of the carbohydrate hydrolases that have been described to date occur in the membrane as homodimers.
The enzymatic activities involved in brush border hydrolysis include sucrase, isomaltase, glucoamylase, and lactase (more properly referred to as lactase/phlorizin hydrolase based on another enzymatic activity expressed by this specific protein). Sucrase and isomaltase activities are actually encoded in a single polypeptide chain with two distinct active sites, and thus the complete protein is referred to as sucrase-isomaltase. Overall, the brush border hydrolases cooperate to facilitate the complete digestion of dietary carbohydrates and the products derived from their luminal digestion.
Considerable information has been obtained about the structure and function of sucrase-isomaltase, which has provided a useful model of the gene regulatory events that accompany the differentiation of enterocytes from secretory cells in the crypt to the absorptive phenotype seen on the villus tips. This enzyme is expressed throughout the small intestine, but not in the stomach or colon, and complex regulatory factors govern its expression along both the vertical and horizontal axes of the intestine. Expression levels are higher in the villus than the crypt, and higher in the proximal than in the distal small intestine. Similar regulatory mechanisms likely also govern the expression of other brush border hydrolases, not only for carbohydrates, but also for peptides as will be discussed later. The sucrase-isomaltase proform is inserted into the apical plasma membrane of enterocytes where it becomes exposed to pancreatic trypsin, which cleaves the molecule to two segments that are then held together by noncovalent forces. The enzymatic activity of the two domains is quite distinct. Thus, while we will learn later that the isomaltase active site is critical for digesting the α-1,6 bonds contained in α-limit dextrins, it is incapable of digesting the α-1,2 bond that links the two monosaccharides contained in sucrose, namely glucose and fructose.
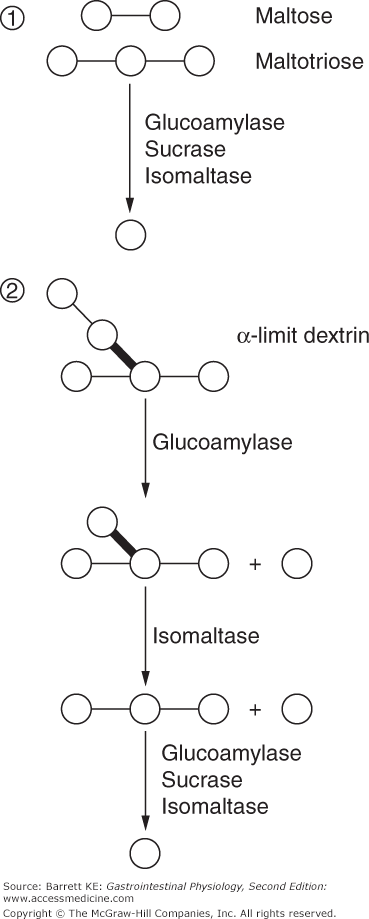
The final digestion of these products of amylose and amylopectin digestion involves the concerted action of several brush border enzymes (Figure 15–2). Glucoamylase, sucrase, and isomaltase activities are all capable of digesting the bonds contained in linear, short-chain (2–9 sugar units) oligomers of glucose, which include maltose and maltotriose. The relative contribution of each enzyme to digestion of these substrates is not known. However, isomaltase is critical for the full digestion of starch, since it is unique among the listed activities in being able to cleave not only the α-1,4 bonds of linear glucose oligomers, but also the α-1,6 bonds of the α-limit dextrins. Glucose released by the brush border hydrolysis of any of these substrates, however, uses a common mechanism to enter the enterocyte cytosol. Uptake is mediated by SGLT-1, a sodium–glucose cotransporter that we previously encountered in Chapter 5. This cotransporter takes advantage of the low intracellular sodium concentration established by the basolateral Na,K-ATPase to accumulate glucose in the cytosol against a concentration gradient (i.e., uphill transport). Additional discussion of the molecular characteristics of SGLT-1 and its regulation will be provided later.
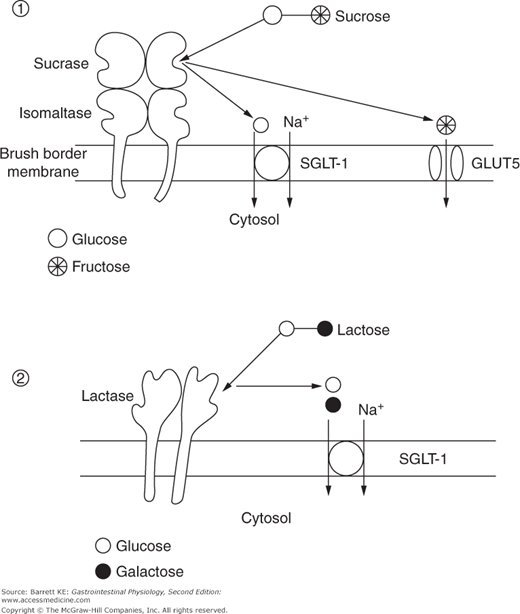
Sucrose, or table sugar, is a prominent carbohydrate in many western diets and requires no luminal digestion because it is a simple disaccharide consisting of glucose and fructose. Rather, it is digested exclusively at the level of the brush border by the enzyme sucrase, yielding the respective monosaccharides (Figure 15–3). As noted previously, sucrase represents one active site of the bifunctional hydrolase, sucrase-isomaltase. Expression of sucrase-isomaltase is usually significantly in excess of the requirements for this enzyme, at least in western populations that emphasize sucrose in the diet. This means that the rate-limiting step for sucrose assimilation is not its hydrolysis, but rather the uptake of the released products across the apical membrane of the enterocyte. This is particularly the case for fructose, which enters the cytosol not via the SGLT-1 transporter used by glucose, but rather a sodium-independent, facilitated diffusion pathway known as GLUT5.
Lactose is an important nutrient in those who consume large quantities of milk, and thus its assimilation is predictably important in infants. Lactose is a disaccharide that consists of glucose and galactose, and provides an energy source for the developing infant that can easily be digested and absorbed. Lactose is broken down at the brush border by lactase, an enzyme that contains two identical active sites within a single polypeptide chain. The products of this hydrolysis reaction are, in turn, both substrates for SGLT-1 and thus can be accumulated against a concentration gradient (Figure 15–3).
Lactose assimilation is limited in two important ways. First, there is a developmental decline in lactase expression, meaning that levels of this enzyme in adulthood may be inadequate to hydrolyze all of the substrate presented to them. Thus, in contrast to the assimilation of sucrose, lactose hydrolysis, rather than transport of the products of this reaction, is usually rate-limiting for the assimilation of this disaccharide. Second, the activity of lactase is inhibited by glucose, in a process known as “end-product inhibition.” If glucose levels rise in the vicinity of the enzyme, breakdown of lactose will further be inhibited. These factors likely contribute to the relatively high prevalence of a condition known as “lactose intolerance,” which will be discussed in more detail later.
As a monosaccharide (as opposed to its presence in sucrose), fructose was previously not a common constituent of most diets. However, its consumption is increasing rapidly with the use of high-fructose corn syrups in food manufacturing. It is also included in so-called “sugar-free” candies prepared for diabetics, which are sweetened predominantly with this sugar. Such candies are not noncaloric, or even low in calories, but are useful because they avoid a rapid increase in plasma glucose that occurs when candies sweetened with either sucrose or glucose are eaten. However, ingestion of large quantities of fructose is likely to overwhelm the limited capacity of GLUT5 in the brush border membrane, meaning that the unabsorbed sugar continues into the large intestine where it is acted on by bacterial enzymes. The symptoms that occur in this setting are comparable to those encountered when a lactose-intolerant individual consumes dairy products.
The final steps in carbohydrate assimilation involve specific membrane transport pathways that permit uptake of these hydrophilic molecules across the enterocyte apical membrane, as well as mediating their transfer out of the enterocyte across the basolateral membrane and thence into the portal circulation. We have already discussed two of these transporters, SGLT-1 and GLUT5. SGLT-1 is of broader significance because it was the first mammalian transport protein to be cloned, by Ernest Wright at UCLA. It is synthesized by villus enterocytes but not by those in the crypts, likely as a result of transcriptional regulatory mechanisms that parallel those involved in establishing brush border hydrolase expression. It exists in the membrane as a homotetramer, which appears to be important for its function. The protein mediates the ordered transfer of both sodium and glucose across the membrane. Sodium binds first to an extracellular site on the transporter, followed by glucose, which triggers a conformational change in the protein (Figure 15–4). This transfers these substrates to the cytoplasmic face of the membrane, where first glucose, then sodium, can dissociate into the cytosol. Recent evidence suggests that the transport cycle of this protein likewise transfers significant numbers of water molecules along with the sodium and glucose, as discussed in greater detail in Chapter 5.