Fig. 10.1
Schematic view of the CGRP receptor that consists of calcitonin receptor-like peptide (CLR), receptor activity modifying protein 1 (RAMP1), and receptor component protein (RCP)
The human C-terminal fragment of the peptide, hCGRP8–37, was the first and widely used tool for determining whether the action of CGRP as well as that of CT is mediated via specific CGRP receptors or CT receptors. It selectively antagonizes CGRP activity, being also an agonist for CT receptor (van Rossum et al. 1997). So far some CGRP antagonists are in development as a potential treatment for acute migraine attacks (Negro et al. 2012). This new class of drugs is designed to block and/or reverse CGRP-mediated dilation of intracranial vessels induced by activation of the main sensory nerves in the brain. CGRP is also thought to play a role in cardiovascular homeostasis and nociception (Wimalawansa 1996; van Rossum et al. 1997).
10.2 Distribution
CGRP is widely distributed in both peripheral and central neurons. The function and expression of CGRP may differ depending on the location of synthesis in the spinal cord. CGRP is derived mainly from the cell bodies of motor neurons when synthesized in the anterior horn of the spinal cord and may contribute to the regeneration of nervous tissue after injury. When synthesized in the posterior horn of the spinal cord, CGRP came from posterior root ganglion and may be linked to the transmission of pain. In the trigeminal vascular system, the cell bodies on the ganglion are the main source of CGRP. High levels of CGRP-like immunoreactivity (LI) have been found in the rat (Sternini et al. 1987) and human (Tani et al. 1999) stomach and the absence of CGRP-LI in endocrine cells suggests that it is more important as a neurotransmitter than as a circulating hormone. Further to dorsal root ganglia, gastric muscle, and mucosa are innervated by vagal afferent from nodose ganglia, another source of CGRP playing important functional role of the stomach (Young et al. 2008) (Fig. 10.2). Since no positive CGRP cells are found in the stomach, most gastric CGRP is of extrinsic origin and is contained and released from peripheral endings of capsaicin-sensitive afferents (Holzer 2007).
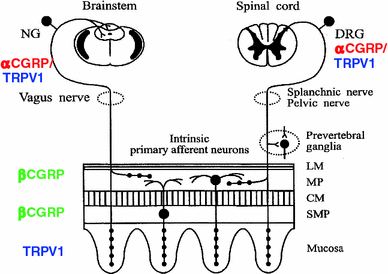

Fig. 10.2
Schematic representation of the extrinsic and intrinsic CGRP-containing neurons and TRPV-1 distribution in the gastrointestinal tract. Extrinsic spinal afferents have their cell bodies in the dorsal root ganglia and contain only α-CGRP. CGRP-containing extrinsic nerve terminals innervate the circular and longitudinal smooth muscle layers, the myenteric plexus and the submucosal plexus, where they densely innervate vascular structures and the mucosa. CGRP-containing intrinsic enteric neurons express only β-CGRP that are localized in the submucosal and myenteric plexuses. Submucosal neurons innervate the mucosa, whereas myenteric neurons project orally and aborally, both within the myenteric plexus and to the circular muscle layer. TRPV-1 was found in both DGR and NG and in the latter coexpressed with CGRP only in 10 % of the neurons. TRPV-1 is present also in the parietal cells of the mucosa. CM circular smooth muscle; DRG dorsal root ganglion; LM longitudinal smooth muscle; MP myenteric plexus; NG nodose ganglia; SMP submucosal plexus
In the enteric nervous system CGRP-β is expressed in a subset of Dogiel type II, intrinsic primary afferent neurons that are not sensitive to capsaicin, while CGRP-α is the primary isoform expressed in the sensory afferent neurons of the extrinsic nervous system originating from the dorsal root and vagal ganglia (Fig. 10.2).
10.3 Target of TRPV-1 Agonists and Antagonists
Capsaicin is the main capsaicinoid in chili peppers, followed by dihydrocapsaicin. These two compounds are also about twice as potent to the taste and nerves as compared to the minor capsaicinoids such as nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin (Table 10.1). Capsaicin is believed to be synthesized in the interlocular septum of chili peppers by addition of a branched-chain fatty acid to vanillylamine; specifically, capsaicin is made from vanillylamine and 8-methyl-6-nonenoyl CoA. Biosynthesis depends on the gene AT3, which resides at the pun1 locus, and which encodes a putative acyltransferase. Besides the six natural capsaicinoids, the synthetic capsaicinoid vanillylamide of n-nonanoic acid (VNA, also PAVA) is used as a reference substance for determining the relative pungency of capsaicinoids (Table 10.1).
Table 10.1
Names and characteristics of the natural capsacinoids and the synthetic one (PAVA) used as reference for pungency
Capsaicinoid name | Abbreviations | Typical relative amount (%) | Chemical structure |
|---|---|---|---|
Capsaicin | C | 69 | 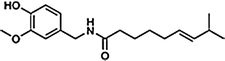 |
Dihydrocapsaicin | DHC | 22 | 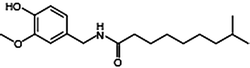 |
Nordihydrocapsaicin | NDHC | 7 | 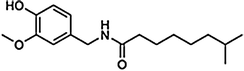 |
Homodihydrocapsaicin | HDHC | 1 | 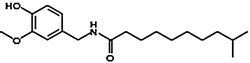 |
Homocapsaicin | HC | 1 | 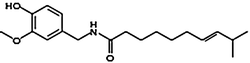 |
Nonivamide | PAVA | 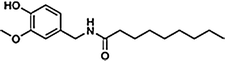 |
From the pioneering work of Jancsò (1960) it has been shown that capsaicin is a selective neurotoxin for sensory nerves, and this has led the exploration of the functional role of these neurons, e.g., at the gastrointestinal level and to the hypothesis of dual “sensory-efferent” functions mediated by capsaicin-sensitive neurons (Holzer 1991, 2007). Capsaicin affects the afferent neurons by two distinct phases: an initial excitation followed by a long-lasting refractory state during which the neurons are not responsive to the neurotoxin and other stimuli. The refractory state (also called desensitization) is usually reversible in terms of weeks, but the systemic administration of high doses of the neurotoxin causes permanent neurotoxicity (Holzer 1991). These neurons are important to transmit sensory information toward the central nervous system but also to release their neurotransmitters toward the periphery, playing a local effector role in several regulatory processes. CGRP is considered the likely mediator of sensory neurons in the stomach and their marker in the upper gastrointestinal tract. Recent studies have shown that capsaicin specifically targets these afferent nerves via transient receptor potential vanilloid of type-1 (TRPV-1), a non selective cation channel with high permeability for Ca++ (Holzer 2007). TRPV-1 belongs to a family of store operated Ca++ channels and acts as a polymodal detector of potentially harmful stimuli including capsaicin, resiniferatoxin, noxious heat, H+ ions, anandamide, and several lipoxygenase products (Holzer 2007). Gingerol, piperine, and zingerone are other spices that might act as TRPV-1 agonists. TRPV-1 and CGRP coimmunoreactivity was found in almost all the dorsal root ganglia and in 10 % of the nodose ganglia (Zhong et al. 2008; Fig. 10.2).
The dual “sensory-efferent” functions can be envisaged with gastric ulcers: animals pretreated with high doses of capsaicin show an aggravation of experimentally induced ulceration because of depletion of protective neurotransmitters. On the other hand, local application of low doses of capsaicin affords gastric protection through the release of neuropeptides (among them: substance P and CGRP) in the periphery (Holzer 1991, 1998; Abdel-Salam et al. 1999; Szolcsànyi and Bartho 2001; Evangelista 2009). This was confirmed by the use of agonists and antagonists of the TRPV-1 (Peng and Li 2010). Functional TRPV-1 antagonists such as ruthenium red and capsazepine produced aggravation of gastric antral ulcers and inhibited the gastroprotective effects of low doses of capsaicin (Yamamoto et al. 2001). Resiniferatoxin is a naturally occurring, ultrapotent capsaicin analog that activates the vanilloid receptor in a subpopulation of primary afferent sensory neurons involved in nociception. Resiniferatoxin causes an ion channel in the plasma membrane of sensory neurons to become permeable to cations, most particularly the calcium cation; this evokes a powerful irritant effect followed by desensitization and analgesia. Neurochemical and functional studies were carried out to investigate and to compare the effects of resiniferatoxin and capsaicin in the rat stomach (Tramontana et al. 1994). Neonatal administration of resiniferatoxin (0.6–1.6 μmol/kg s.c.) produced a marked decrease in gastric CGRP-LI in both secretory and nonsecretory region of the stomach. Almost complete depletion of the peptide was determined by neonatal administration of capsaicin (164 μmol/kg s.c.). Vasoactive intestinal polypeptide-LI was concomitantly unaffected by resiniferatoxin or capsaicin, thus showing the selectivity of action of the neurotoxins on gastric afferent fibers. Oral administration of an equimolar dose (0.3 nmol/kg) of resiniferatoxin or capsaicin together with 50 % ethanol reduced at similar extent gastric haemorrhagic lesions produced by the mucosal barrier-breaker agent. These findings provide evidence that resiniferatoxin and capsaicin may act on a common neuronal target in the rat stomach and that the acute exciting (protective) effect is of the same magnitude (Mòzsik et al. 2006). In order to study compounds less pungent than capsaicin, a group of analogs named capsinoids (the natural capsiate, dihydrocapsiate, dihydrocapsiate, and the synthetic capsiate analog vanillyl nonanoate) with similar chemical structure of lead compound and TRPV-1 agonistic properties were developed (Luo et al. 2011). Both capsiate and its analog vanillyl nonanoate protected rat gastric mucosa from ethanol-induced injury by the release of CGRP and this effect was reverted by prior administration of the TRPV-1 antagonist capsazepine (Luo et al. 2011; Li et al. 2012). The gastroprotection of capsinoids was parallel to a decrease in cellular apoptosis and caspase-3 activity and to an increase of antioxidant activity seen as superoxide dismutase and malondialdehyde activity. Rutaecarpine, an alkaloid found in certain herbs including Evodia rutaecarpa, protects the gastric mucosa against injury induced by ASA and stress, and its gastroprotective effect is related to a stimulation of endogenous CGRP release via activation of the vanilloid receptor being reverted by pretreatment with capsaicin or capsazepine (Wang et al. 2005). Another TRPV-1 agonist such as anandamide was found able to protect gastric mucosa from injury induced by restrain stress in rats, and this effect was partially mediated by sensory nerves (Warzecha et al. 2011).
10.4 Biological Actions
CGRP, being a very potent vasodilator, is able to produce positive inotropic activity and vasorelaxation. Due to its wide distribution in the gastrointestinal tract CGRP has been the target of many studies summarized in some reviews (Holzer 1992, 2007; Bartho et al. 2008; Evangelista 2009). CGRP-containing pathways of the enteric nervous system have been shown to be involved in intestinal nociception, modulation of gastrointestinal motility and secretion (Fig. 10.3).
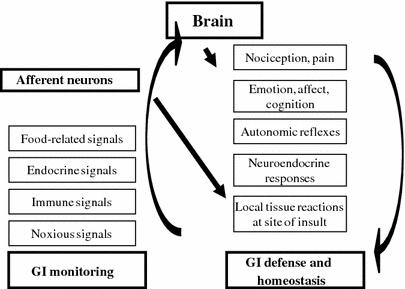

Fig. 10.3
Role of afferent neurons in gastrointestinal (GI) defense. The figure shows how primary afferent neurons monitor the chemical and physical environment within the GI tract and the various modalities of the response in order to maintain homeostasis: via brain-mediated reactions and reflexes and through local neuropeptides release at the site of the insult
As regards the motility, two distinct effects appear important for CGRP action on intestinal smooth muscle: a direct relaxant effect, observed in the longitudinal and circular muscles of intestinal segments from various species and an indirect effect, produced via stimulation of neuronal receptors leading to the release of other transmitters which, in turn, affect intestinal motility (Bartho et al. 2008). The human gut (especially the circular musculature) is relaxed by capsaicin, but this effect seems to be under the control of different neurotransmitter agents, nitric oxide (NO), and VIP, neither of them of intrinsic neuronal origin. The “local efferent” effect of extrinsic afferent neurons can also be demonstrated in vivo, both in animals and man. Yet, nearly normal motility of the small and large intestines is maintained in animals with functionally inhibited capsaicin-sensitive nerves. CGRP is involved also in the peristaltic reflex elicited by muscle stretch and mucosal stimulation (Grider 1994). Peristaltic reflex are subjected to the activity of several neurotransmitters: both the 5-HT4 receptor antagonists and the CGRP antagonist hCGRP8–37 inhibit ascending contraction and descending relaxation. Thus, the reflex in mouse, like that in rat and human intestine, is initiated by mucosal release of 5-HT and activation of 5-HT4 receptors on CGRP sensory neurons and is relayed via somatostatin and opioid interneurons to VIP/nitric-oxide synthase inhibitory motor neurons and via cholinergic interneurons to acetylcholine/tachykinin excitatory motor neurons. The importance of this system in regulating GI movements may be exaggerated under pathophysiological conditions, first of all inflammation. The afferent function of capsaicin-sensitive nerves plays a role in sympathetic reflexes, such as the inhibition of GI motility after laparotomy or by peritoneal irritation and diabetic gastroparesis (Bartho et al. 2008).
CGRP is a potent suppressor of basal and secretagogues-stimulated gastric acid and pepsin secretion in humans, dogs, rabbits, and rats (Ren et al. 1998; Evangelista 2009). Multiple mechanisms seem to be involved in the CGRP antisecretory activity such as the release of somatostatin from D cells (Ren et al. 1998), where CL and RAMP1 mRNAs has been found, the direct inhibition of gastrin- and cholinergic-dependent stimulatory effects and the stimulation of mucus and bicarbonate from gastroduodenal mucosa (Evangelista 2009).
Concerning the ion and fluid intestinal transport, CGRP exhibited concentration-dependent dual actions upon rat colonic epithelia acting directly on colonic enterocytes. At low concentrations (i.e., 0.03–10 nM) CGRP produces antisecretory effects that are abolished by pretreating tissues with tetrodotoxin (TTX). At higher concentrations (30–200 nM) secretory responses predominate, and these effects were not abolished by TTX (Cox et al. 1989). In guinea-pig colon, CGRP depolarizes myenteric neurons that are synaptically coupled to submucosal neurons. Cholinergic neurons using nicotinic and muscarinic synapses for transmission of information to the epithelium are involved in inducing chloride secretion. CGRP is also present in human epithelia but it is not known whether the described secretory effects are of physiological relevance. It should be observed that in human volunteers the intraluminal capsaicin application did not affect electrolyte absorption and fluid secretion (Hammer et al. 1998). During inflammation the activation of CGRP receptors may result in effects on tight junction permeability not observed in normal tissue (Holzer 2007).
It is known that the vascular system of the gastrointestinal tract is innervated by both sympathetic vasoconstrictor and parasympathetic vasodilator neurons but splanchnic blood vessels receive also a dense supply of fibers containing CGRP (Sternini et al. 1987; Green and Dockray 1988). These fibers innervate blood vessels of the entire body and their density is greater around arteries than veins. In the gastrointestinal tract, CGRP-containing nerve fibers are surrounding the mucosa and submucosa blood vessels and species differences are known concerning their presence, being scarce in human and porcine stomach (Green and Dockray 1988). CGRP exerts also a potent vasodilator and past studies have shown it as a noncholinergic and nonadrenergic transmitter able to dilate mesenteric artery after periarterial stimulation (Holzer 1992). When released from extrinsic afferent nerve fibers in acid-threatened stomach, CGRP causes a NO-dependent vasodilation that has been shown to be a defensive signal in the face of pending acid injury in the gastric mucosa (Holzer 1998) (Fig. 10.3). The vasodilator effect of CGRP has been widely studied in the upper gastrointestinal tract (see below) and is working under pathological conditions.
10.5 Pathophysiology
10.5.1 CGRP in Gastrointestinal Defence
10.5.1.1 Experimental Evidences
Both indomethacin- or ethanol-induced mucosal lesions were enhanced by systemic administration of hCGRP8–37 while low dose of capsaicin and/or TRPV-1 agonists have clearly shown to exert gastroprotection via the stimulation of sensory nerves and the local release of CGRP (Holzer 2007) indicating that endogenous CGRP plays a trophic role on mucosal defense of the stomach. Further, gastroprotection by capsaicin was dose-dependently antagonized by the CGRP antagonist, hCGRP8–37, and by a monoclonal antibody to CGRP (Evangelista 2009). Recent studies using CGRP-knockout mice (Ohno et al. 2008) have strongly confirmed these findings: the protective action of capsaicin against ethanol-induced lesions was completely abolished in CGRP−/− mice (Fig. 10.4). The authors also showed that the adaptive gastroprotection induced by previous exposure of the stomach to mild irritants was mediated by CGRP being completely absent in CGRP knockout mice (Ohno et al. 2008).
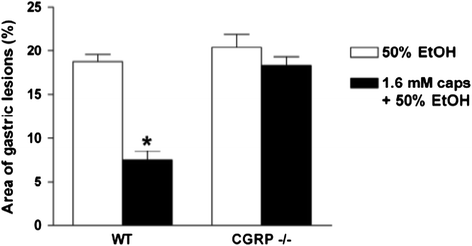

Fig. 10.4
Lack of capsaicin (caps)-induced gastric mucosal protection against 50 % ethanol-induced lesions in mice knockout to CGRP (CGRP−/−). Means ±S.E values of 5–6 mice for group. * = P < 0.01 between wild type (WT) groups. Modified from (Ohno et al. 2008)
Furthermore, several studies have reported a depletion of tissue CGRP-LI during the formation of several gastric ulcers (Evangelista 2009). Decreased levels of gastric CGRP-LI were observed after acetic acid-, cysteamine-, concentrated ethanol- or water-immersion stress-ulcers, and the ulcerogens did not affect the tissue content of other peptides (Evangelista 2009) suggesting that reduction in gastric CGRP-LI cannot be attributed only to damage of the tissue.
On the other hand, restoration of CGRP-LI was observed in animals bearing ulcers during healing (Evangelista 2009), and intense CGRP immunostaining was observed in the ulcer margin of patients with gastric ulcers (Tani et al. 1999). Healing of ulcer elicited by acetic acid in CGRP knockout mice was markedly delayed as compared with wild type mice as well as the granulation tissue, and neovascularization at the base of these ulcers was poor in CGRP−/− animals (Ohno et al. 2008). Furthermore, in isolated rat stomachs exposed to hyperosmolar concentrations of sodium chloride, the addition of CGRP enhanced the recovery of mucosal integrity after mild but not severe damage produced by the lesioning agent (Miller et al. 1995). These findings and the observation that there is a tonic flow of CGRP toward the peripheral endings of sensory fibers suggest that the peptide is restored in connection with the healing of ulcers after its release produced by the ulcerogen.
As regards the known CGRP antisecretory effect (Evangelista 2009) along with somatostatin releasing activity from gastric D cells (Ren et al. 1998), other mechanisms have been explored to explain the CGRP protective role in the stomach. It is known that hyperemia induced by an injury is a physiological response to tissue damage and limits the extent of injury in the stomach (Holzer 1992, 1998). Modulation of this response is an important defense mechanism and it has been reported that CGRP released by capsaicin-sensitive fibers is involved in gastric hyperemic protective effects (Holzer 1998).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


