Study, duration
n
Age (years) Sex (M:F)
OA site
Formulation
Outcomes
Rate of application site burning
Deal 1991 (Deal et al. 1991) 4 weeks
70
61 25M:45F
Knee
0.025 % capsaicin
VAS pain −13.9 %, p = 0.061
23/52 (44 %) capsaicin; 1/49 (0.02 %) placebo patientΩ
Pain (categories) −0.35, p = 0.053
Global evaluation −0.39, p = 0.051
McCarthy 1992 (McCarthy and McCarty 1992) 4 weeks
14
65 ± 2 5M:9F
Hand
0.075 % capsaicin
Pain VAS ~−40 % (p < 0.02)
7/7 capsaicin; 0/7 placebo
Tenderness scores ~ −30 % p < 0.02. No effect on grip strength, joint swelling, duration of morning stiffness or function
Altman 1994 (Altman et al. 1994) 12 weeks
113
62 ± 12¥
Knee
0.025 % capsaicin
Pain VAS Significant differences by week 4, effect size −9 mm at week 12
26/36 (46 %) of capsaicin users
Tenderness Less tenderness in capsaicin group by week 8 (p = 0.03)
Global assessment (physician) significant improvement by week 4 (p = 0.042). Patient assessment by week 4 (p = 0.023)
Pain severity (categorical) and HAQ: no differences
McCleane 2000 (McCleane 2000) 5 weeks
200
~49 ± 14 78M:89F
Hip, knee, shoulder, hand
0.025 % capsaicin ± 1.33 % GTN
VAS pain scores Difference between GTN + capsaicin and GTN or capsaicin alone p < 0.05
Not reported
Analgesic use Difference between any actives and placebo p < 0.05, Adding GTN to capsaicin has additive effect. GTN application reduced burning sensation when combined with capsaicin
Schnitzer 2012 (Schnitzer et al. 2012) 12 weeks + extension
695
61 33 %:67 %
Knee
0.075 % civamide Placebo 0.01 % civamide
Primary outcomes (12 weeks) using TWA WOMAC pain p = 0.009. WOMAC physical function: p ≤ 0.001
35 % in capsaicin group, 11 % in placebo p ≤ 0.001
Subject global evaluation p = 0.008. Pain and physical function effect sizes greater in patients with higher pain scores
Kosuwon 2010 (Deal et al. 1991) 4 weeks treatment
70
61 (44–82) years 0M:61F
Knee
0.025 % capsaicin
VAS pain−0.72, p ≤ 0.05
66.6 % of patients during the capsaicin phase versus 16.6 % patients during placebo phase; p ≤ 0.05
WOMAC pain −3.42, p ≤ 0.05
WOMAC function −8.97, p ≤ 0.05
Studies included in Table 11.1 assess the effect of treatment with capsaicin (or capsaicin–like compounds) for 4–12 weeks in older adults with OA of the knee (Deal et al. 1991; Schnitzer et al. 2012; Kosuwon et al. 2010), hand (McCarthy and McCarty 1992), and a mixture of joints (Altman et al. 1994; McCleane 2000). Inclusion and exclusion criteria varied between trials but patients were typically required to have at least moderate pain and either radiological evidence of OA or be clinically diagnosable as having OA, or both.
11.6 Treatment Efficacy Within Placebo–Controlled Trials
Studies described in Table 11.1 show the efficacy of capsaicin (vs. placebo) on a range of outcomes, including pain using a visual analog scale (VAS), either a 0–100 scale or a 0–10 scale, as well as other outcomes including Western Ontario and McMaster Universities Index (WOMAC) pain, function and stiffness scales, patient and physician global evaluation, tenderness, grip strength, joint swelling, duration of morning stiffness, analgesic use, and adverse events.
VAS pain is the most commonly assessed outcome, and therefore we have further summarized the effect of capsaicin on pain intensity as assessed by visual analog scores (VAS) in Table 11.2. VAS scores have been compared using standardized mean differences in studies with available data (Deal et al. 1991; McCarthy and McCarty 1992; Altman et al. 1994; McCleane 2000; Kosuwon et al. 2010), using Review Manager (Revman) version 5.2 (The Cochrane Collaboration, Copenhagen). By convention (Cohen 1988), the standardized mean difference values of 0.2, 0.5, and 0.8 are considered small, medium, and large. Table 11.2 shows that capsaicin has a medium sized effect (SMD = 0.44) on pain intensity in patients with painful OA over 4 weeks of treatment. Results were remarkably consistent across trials with no heterogeneity, suggesting no dose response from different doses of capsaicin, and no difference in the effect of capsaicin on OA of different sites (e.g. hand vs. knee).
Table 11.2
Forest plot comparing changes in VAS pain score over 4 weeks of treatment with capsaicin in patients with painful osteoarthritis
Study or subgroup | Capsaicin | Placebo | Weight (%) | Std. mean difference | Year | Std. mean difference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
Mean | SD | Total | Mean | SD | Total | IV random, 95 % CI | IV random, 95 % CI | |||
Deal 1991 | 33.4 | 18 | 36 | 19.5 | 23 | 34 | 14.3 | 0.67 [0.19, 1.15] | 1991 | 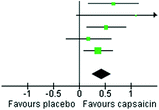 |
Mc Carthy 1992 | 55 | 31.7 | 7 | 19 | 29.1 | 7 | 2.5 | 1.11 [−0.05, 2.26] | 1992 | |
Altman 1994 | 29 | 15.1 | 57 | 20 | 18.7 | 56 | 23.7 | 0.53 [0.15, 0.90] | 1994 | |
McCleane 2000 | 0.49 | 2.37 | 40 | 0.08 | 2.05 | 40 | 17.3 | 0.18 [−0.26, 0.62] | 2000 | |
Kosuwon 2010 | 2.04 | 1.93 | 99 | 1.31 | 1.97 | 99 | 42.2 | 0.37 [0.09, 0.65] | 2010 | |
Total (95 % CI) | 239 | 236 | 100.0 | 0.44 [0.25, 0.62] | ||||||
Heterogeneity Tau2 = 0.00; Chi2 = 3.88, df = 4(P = 0.42); I 2 = 0 % | ||||||||||
Test for overall effect Z = 4.69 (P < 0.00001) | ||||||||||
Most studies report capsaicin use over 4 weeks, with only, two studies continued treatment beyond 4 weeks (Altman et al. 1994; Schnitzer et al. 2012), both of which reported that continuing use was associated with beneficial effects. The study reported by Altman et al. (1994) demonstrated that the maximum difference between groups occurred at 4 weeks, and differences between groups remained similar between weeks 4 and 12. Schnitzer et al. (2012) report that differences in groups increase over time, even up to 20 weeks. These studies demonstrate that treatment with capsaicin is associated with benefits for treatment periods exceeding 4 weeks but that 4 weeks is probably sufficient for a therapeutic trial.
Two studies reported assessments of how patients reported the overall impression of treatment effectiveness (symptoms better, much better or completely gone) after 4 weeks. Capsaicin treated patients had a 40 % increased likelihood of reporting that their symptoms were at better after 4 weeks of treatment (Table 11.3). Schnitzer’s study reported that after 12 weeks, the Osteoarthritis Research Society International (OARSI) simplified response was 10 % greater in civamide-treated patients (Schnitzer et al. 2012), suggesting that response to treatment continues up until at least 12 weeks.
Table 11.3




Forest plot of patient global evaluation of treatment effectiveness after 4 weeks of treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


