Fig. 9.1
Inhibition of gastric acid basal output (BAO) by capsaicin in 16 healthy human subjects. (After Mózsik et al. 2005)
9.3.2 Affinity and Intrinsic Affinity Curves for the Capsaicin, Muscarinic (Atropine, Pirenzepine), H2-Receptor Antagonists (Cimetidine, Ranitidine, Famotidine), and Proton Pump Inhibitors (Omeprazole and Esomeprazole) on the Gastric BAO in Healthy Human Subjects
The action of the compounds inhibiting the gastric basal acid secretion is shown in Fig. 9.2. The curve indicates that no competitive actions of these drugs exist on the gastric basal acid output. The pD2 values were calculated from the affinity curves obtained in the molecular pharmacological studies.
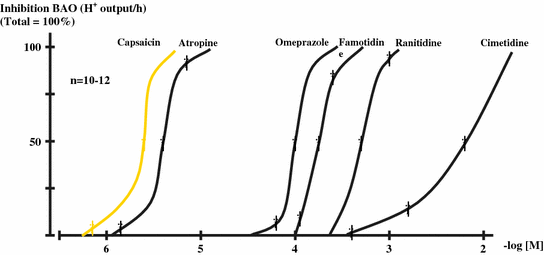

Fig. 9.2
Affinity curves for drugs inhibitory actions of different antisecretory drugs on the BAO in healthy human subjects. The absolute values were calculated as H+ output/h, the presentations of curves expressed in percent value (total = 100 %) (mean ± SEM). (After Mózsik et al. 2007)
The intrinsic activity curves for the drugs inhibiting the gastric basal acid outputs in healthy human subjects were calculated. The intrinsic activity (αatropine = 1.00) was taken to be equal to 1.00, and the values for other drugs were expressed to action of atropine (Fig. 9.3). The pA2 values (50 % inhibition of intrinsic activity in (−) molar values) were calculated from the intrinsic activity curves.
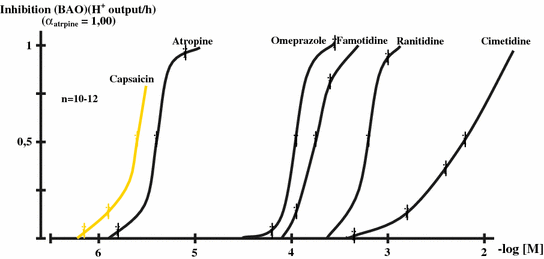

Fig. 9.3
Intrinsic activity curves for the inhibitory drugs of different antisecretory drugs and capsaicin (given in stimulatory doses of capsaicin-sensitive afferent nerves) on the BAO in healthy human subjects, which were expressed to action of atropine (1.00) (α atropine) (mean ± SEM). (After Mózsik et al. 2007)
For the molecular pharmacological understanding the background of the action of drugs, action the molecular weights, pD2 values, of the intrinsic activity (in comparison to atropine action) and pA2 values were calculated and shown in Table 9.1.
Table 9.1
Summary of the affinity (pD2) and intrinsic activity (expressed in value of αatropine = 1.00)(pA2) values of capsaicin, atropine, Pirenzepine, cimetidine, ranitidine, famotidine, Omeprazole, and Esomeprazole on the gastric basal acid output (BAO) in healthy human subjects. (After Mózsik et al. 2007)
Compounds | M.W. | pD2 | Intrinsic activity | pA2 |
|---|---|---|---|---|
Capsaicin | 305.4 | 5.88 | 0.76 | 5.87 |
Atropine | 289.38 | 5.40 | 1.00 | 5.40 |
Pirenzepine | 424.34 | 3.93 | 0.89 | 3.93 |
Cimetidine | 252.34 | 2.23 | 1.00 | 2.23 |
Ranitidine | 314.41 | 3.33 | 1.00 | 3.33 |
Famotidine | 337.43 | 3.77 | 1.00 | 3.77 |
Nizatidine | 331.47 | 3.34 | 1.00 | 3.34 |
Omeprazole | 345.42 | 3.97 | 1.00 | 3.97 |
Esomeprazole | 345.42 | 3.97 | 1.00 | 3.97 |
9.3.3 Changes in the “Parietal” and “Non-Parietal” Components of Gastric Secretory Responses in Healthy Human Subjects
The measurements of cations (H+, sodium, potassium, calcium, magnesium) and of chloride– offered the possibility to identify the “parietal” and “non-parietal” components of gastric secretion in humans without and with administration of different drugs (or compounds) (Hollander 1934).
Using the Hollander’s method for the calculation and evaluation of measurements of cations, chloride in the gastric juice indicated clearly the significant decrease of “parietal component” (∆ − 18 ± 2 mmol/L, P < 0.001) in association with significant increase of “ non-parietal component” (∆ + 19 ± 2 mmol/L, P < 0.001) component of the gastric secretion after application of 400 μg (given orally) of capsaicin (n = 10) (Mózsik et al. 2005) (Table 9.2).
Table 9.2
Chemical composition of gastric juice without (A) and with (B) application of capsaicin (ED50 = 400 μg) in healthy human subjects
H+ | Na+ | K+ | Ca2+ | Mg2+ | |||||
|---|---|---|---|---|---|---|---|---|---|
A | B | A | B | A | B | A | B | A | B |
43 ± 3 | 25 ± 1 | 73 ± 4 | 89 ± 2 | 13 ± 1 | 8 ± 0.6 | 0.98 ± 0.02 | 0.88 ± 0.01 | 0.49 ± 0.01 | 0.38 ± 0.01 |
P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
100 ± 7 | 58 ± 2 | 100 ± 5 | 122 ± 3 | 100 ± 8 | 62 ± 5 | 100 ± 2 | 90 ± 1 | 100 ± 2 | 78 ± 2 |
“Parietal” component | “Non-parietal” component | Albumin (g/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
A | B | A | B | A | B | ||||
43 ± 3 | 25 ± 2 | 126 ± 4 | 145 ± 4 | 1.24 ± 0.001 | 1,630,002 | ||||
P < 0.001 | P < 0.001 | P < 0.001 | |||||||
100 ± 7 | 58 ± 5 | 100 ± 3 | 115 ± 3 | 100 ± 1 | 131 ± 2 | ||||
9.3.4 Changes in Albumin Level in the Gastric Juice After Capsaicin Administration in Healthy Human Subjects
The albumin concentration increased from 1.24 ± 0.001 g/L versus 1.63 ± 0.02 g/L (P < 0.001; n = 10) after 400 μg capsaicin (i.g. given) application (Mózsik et al. 2005).
9.3.5 Action of Capsaicin on the GTPD in the Healthy Human Subjects
Capsaicin (given ig. in doses of 100, 200, 400, and 800 μg) dose-dependently increased the GTPD alone (∆ value from to baseline 10 (−mV)) (Mózsik et al. 2005).
When we applied capsaicin in double dose (800 μg, intragastrically) then we received the same increase in GTPP and in the same time period (Hossenbocus et al. 1975; Mózsik et al. 2005) (Fig. 9.4).
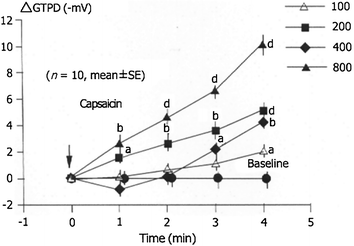

Fig. 9.4
Capsaicin dose-dependent gastric mucosal protective effect of capsaicin on GTPD in 10 healthy subjects. (After Mózsik et al. 2005)
9.3.6 Action of Ethanol on GTPD in Healthy Human Subjects
The intragastrically applied ethanol immediately and significantly decreased the GTPD (∆ 25 (−mV)) (Mózsik et al. 2005).
9.3.7 Preventive Action of Capsaicin on the Ethanol-Induced Decrease of GTPD in Healthy Human Subjects
The intragastrically applied capsaicin (given in doses of 100, 200, 400, and 800 μg) dose-dependently prevented the ethanol-induced decrease of GTPD in healthy human subjects (Mózsik et al. 2003, 2005). 800 μg capsaicin was intragastrically given after each other with 5 min interval (Fig. 9.5).
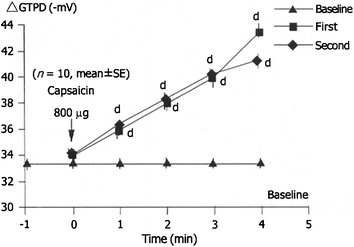

Fig. 9.5
Dose-dependent gastric mucosal protective effect of capsaicin is repeated (800 μg) on the GTPD with 5 min time interval (n = 10, mean ± SEM) in healthy human subjects (after Mózsik et al. 2005; with permission)
9.3.8 Indomethacin-Induced Acute Gastric Microbleeding and the Acute Gastric Mucosal Preventive Effect of Capsaicin on the Indomethacin-Induced Acute Gastric Mucosal Damage in Healthy Human Subjects (Based on the Results of Prospective, Randomized and Multiclinical Study)
9.3.8.1 Extent of Indomethacin-Induced Acute Gastric Mucosal Damage in Healthy Human Subjects
The baseline of blood losing was 2.0 ± 0.2 mL/day (n = 14) without application of IND, which was increased to 8.1 ± 0.2 mL/day (n = 14; P < 0.001).
9.3.8.2 Gastric Mucosal Preventive Effect of Capsaicin on the Indomethacin-Induced Acute Gastric Microbleeding in Healthy Human Subjects
Capsaicin was given in doses of 200, 400, and 800 μg orally before the administration of indomethacin. The acutely applied capsaicin prevented by dose-dependent manner of indomethacin-induced gastric microbleeding in healthy human subjects (Y = −0.0071*X + 7.78; r = −0.98, n = 14; P < 0.001) (Mózsik et al. 2005; Sarlós et al. 2003) (Fig. 9.6).
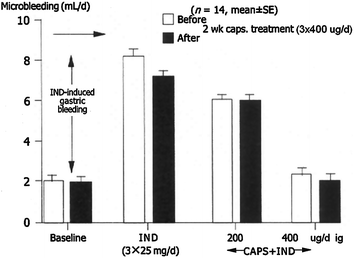

Fig. 9.6
Gastric mucosal protection demonstrated by reduced microbleeding before and after two weeks capsaicin treatment of indomethacin (IND)-induced mucosal damage. (After Mózsik et al. 2005)
9.3.9 Effect of Capsaicin on the Gastric Emptying in Healthy Human Subjects
Capsaicin was intragastrically given in dose of ED50, which increased significantly the gastric acid emptying: (1) Capsaicin (400 μg, ig = ED50)-induced changes in the maximal values of Delta Over Base (DOB max) decreased from 18 ± 1 to 14 ± 1 U (P < 0.01); (2) the time to reach the DOB max decreased from 148 ± 13 to 70 ± 12 min (P < 0.01); (3) the slope (in U/min) increased from 0.11 ± 0.01 to 14 ± 0.001 (P < 0.001); (4) the time to reach the AUC50 decreased from 115 ± 10 to 80 ± 8 (min) (n = 10; P < 0.01) (Debreceni et al. 1999).
9.3.10 Increased Glucose Absorption from Small Intestine and of Glucagon Release by capsaicin During the Glucose Loading Test in Healthy Human Subjects
During glucose loading test we measured the glucose absorption in the proximal part of the small intestine, insulin, C peptide, glycagon using the plasma level of glucose as markers substance (Dömötör et al. 2006a, b) (Figs. 9.7, 9.8).
The absorption of glucose from the small intestine and glucagon release increased by capsaicin administration; however, no significant changes were obtained neither in insulin nor in C-peptide release under these observational circumstances (Fig. 9.7).
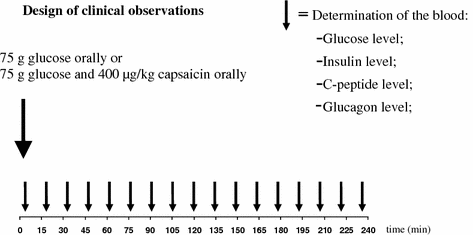
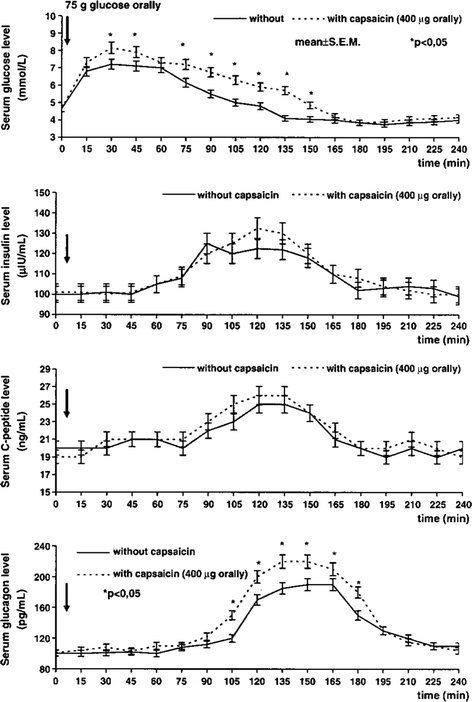

Fig. 9.7
Design of clinical observations with glucose observation and utilization in 14 healthy human subjects without and with (400 μg orally) capsaicin application. The measurement of glucose, insulin, C-peptide, glucagon was carried out from the plasma level in every 15 min period from the glucose application to 4 h. (After Dömötör et al. 2006a, b)

Fig. 9.8
Changes in plasma level of glucose, insulin, C-peptide, and glucagon after oral administration of glucose (75 g in 100 ml water) in 14 healthy human subjects. Capsaicin (400 μg) was orally given in gelatine capsule (Hungaropharma, Budapest, Hungary). The plasma levels of glucose, insulin, C-peptide, and glucagon were measured every 15 min for 4 h. The results are expressed as mean ± SEM. (After Dömötör et al. 2006a, b)
The plasma levels of glucose increased significantly 30–150 min and the plasma level of glucagon increased from 90 to 180 min after capsaicin administration in healthy human subjects given 75 g glucose orally. The plasma levels of insulin and C-peptide increased from 75 to 165 min after glucose administration; however, levels did not differ significantly.
9.3.11 Results of the Immunohistochemical Examinations in the Human Gastric and Large Bowel Mucosa in Healthy Human Subjects and in Patients with Different Gastrointestinal Disorders
9.3.11.1 Demonstration of Capsaicin Receptors, CGRP, and SP in the Human Gastric and Large Bowel Mucosa in Healthy Human Subjects
The results of these observations were obtained in “healthy human subjects,” who had different functional complaints and the endoscopic examinations were carried out to exclude the presence of any histologically proven disease (the clinical histological examinations were carried out by the independent pathologist) and the opinion of pathologist gave normal histology.
The immunohistochemical examination demonstrated the presence of capsaicin (TRVP1) receptors in the gastric and large bowel mucosa obtained from biopsy samples (Fig. 9.8). The location of capsaicin receptor could be demonstrated near the nerve endings, vascular vessel and in the epithelial layer (Fig. 9.9) (Dömötör et al. 2005).
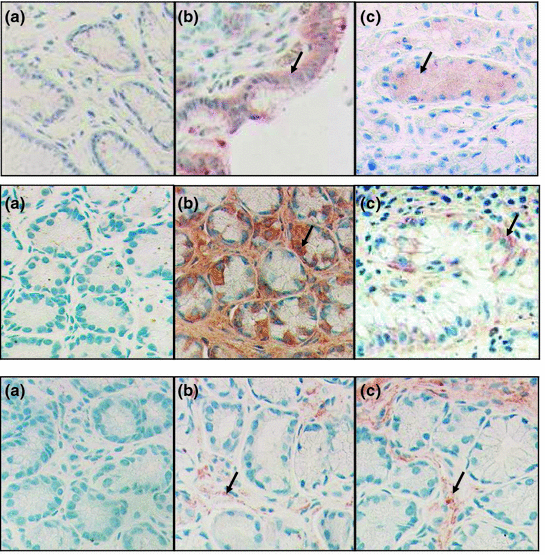

Fig. 9.9
The immune distribution of TRPV1 (first row), CGRP (second row), and of SP (third row) in the gastric mucosa of a healthy subject (a) and of patient with H. pylori negative (b) and H. pylori positive (c) chronic gastritis. Arrows show the immunosigns in the mucosa. (After Mózsik et al. 2007)
The CGRP and SP could be observed in gastric mucosa and these parts of large bowel mucosa as well (Fig. 9.10) (Dömötör et al. 2005).
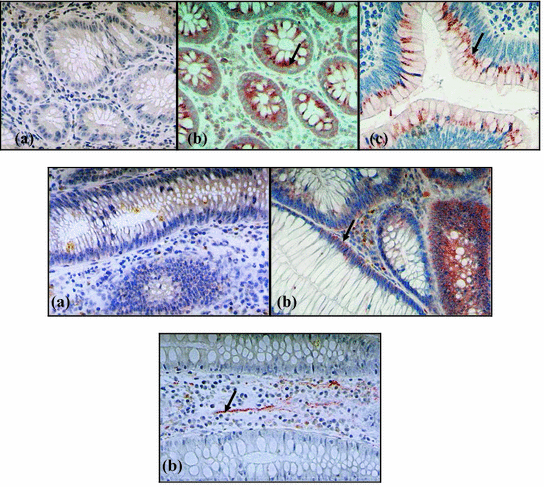

Fig. 9.10
The immundistribution of TRPV1 (first line), CGRP (second line), and of SP (third line) in the colon mucosa of control person (a) and of patient with inflammatory bowel disease (b) and with severe dysplastic polyp (c). Arrows show the immunsigns in the mucosa. (After Mózsik et al. 2007; with permission)
9.3.11.2 Demonstration of Capsaicin Receptor, CGRP, and SP in the Gastric and Large Bowel Mucosa in Patients with Different Disorders
The preliminary results of these observations were published by Dömötör et al. (2005).
These patients went over the clinical endoscopic and pathological examinations. The original pathological diagnosis was given by an independent pathologist.
The capsaicin receptor, and released neuropeptides (CGRP and SP) could be detected in all types of patients with different disorders (Table 9.3). The results of these preliminary clearly indicated the following: (1) capsaicin receptor could be demonstrated in patients with superficial gastritis, erosive gastritis, stomach polyps, stomach cancer, inflammation of large bowel disease, colon polyps with severe dysplasia, and colorectal cancers.
Table 9.3
Immune distribution of capsaicin receptor (TRPV1), calcitonin gene-related peptide (CGRP), and substance P (SP) in the gastric and large bowel mucosa of patients with different GI disorders. (After Mózsik et al. 2007)*
TRPV1 | CGRP | SP | |
|---|---|---|---|
Stomach | |||
Superficial gastritis | + | + | – |
Erosive gastritis | + | + | – |
Gastric ulcer | ± | ± | ± |
Polyp | + | + | – |
Carcinoma | + | + | – |
Large bowel | |||
Inflammation | + | + | + |
Polyp with moderate dysplasia | – | – | – |
Poly with severe dysplasia | +Spot-like | – | – |
Carcinoma | +Spot-like | + | – |
The CGRP could be demonstrated in most of the all of the above-mentioned diseases; meanwhile the SP could not be demonstrated in these diseases (Table 9.3).
9.3.12 Capsaicin Receptor, CGRP, and SP in the Patients with Helicobacter Positive and Negative Chronic Gastritis
These observations were carried out in 57 patients with chronic gastritis (21 patients from them were Helicobacter positive and 30 patients were Helicobacter pylori negative).
9.3.13 Measurements of the Gastric Microbleeding Before and After 2 Week Capsaicin Treatment: Testing of the Changes in Baseline, Indomethacin-Induced Acute Gastric Microbleeding and of Capsaicin-Stimulated Gastric Mucosal Protective Effect on the Indomethacin-Induced Acute Gastric Microbleeding in Healthy Human Subjects
9.3.13.1 Baseline Before and After 2 Weeks Indomethacin Treatment Without Application of any Drug and/or Compound
The baseline of gastric microbleeding was detected 2.1 ± 0.1 versus 2.0 ± 0.1 mL/day, before and after 2 weeks capsaicin treatment (without other drug/compound application).
9.3.13.2 Measurement of Indomethacin-Induced Acute Gastric Mucosal Microbleeding: Before and After 2 weeks Capsaicin Treatment—in Healthy Human Subjects
The acute administration of indomethacin significantly increased the extent of gastric microbleeding versus baseline (without administration of indomethacin) (P < 0.001; n = 14) in healthy human subjects before (baseline vs. Ind., 2.1 ± 0.1 vs. 8.3 ± 0.2 mL/day) and after (baseline vs. Ind., 2.0 ± 0.1 vs. 7.8 ± 0.3 mL/day) 2 weeks capsaicin (3 × 400 μg orally given) treatment (Mózsik et al. 2005).
9.3.13.3 Measurement of Acutely Applied Capsaicin-Induced Gastric Mucosal Protective Effect on the Indomethacin-Induced Acute Gastric Microbleeding Before and After 2 Weeks Capsaicin Treatment in Healthy Human Subjects
The intragastrically applied capsaicin dose-dependently prevented the indomethacin-induced gastric mucosal damage (Mózsik et al. 2003, 2004a, 2005)—before (Y = −0.0087X + 9.18, r = −0.99: P < 0.001) and after (Y = −0.0096X + 10.1; r = −0.99; P < 0.001) 2 weeks capsaicin treatment—when capsaicin was acutely and intragastrically (in doses of 200 and 400 μg) given before the 1 day indomethacin treatment (see the description in the methods) (Mózsik et al. 2005) (Figs. 9.11 and 9.12).
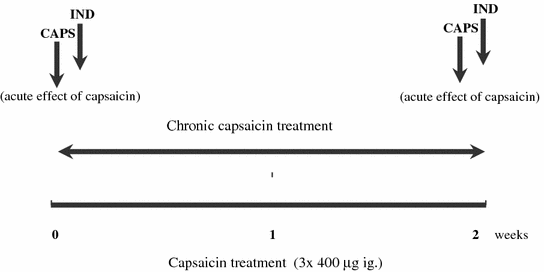
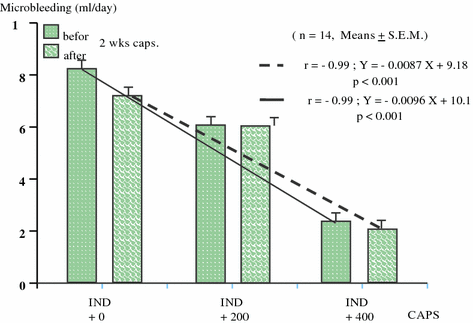

Fig. 9.11
Clinical study design of a chronic capsaicin treatment in healthy human subjects. Abbreviations: CAPS capsaicin, IND indomethacin

Fig. 9.12
Capsaicin (given 200 and 400 μg ig.)-induced gastric mucosal preventive effects on indomethacin (3 × 25 mg ig.)-produced gastric mucosal microbleeding before and after a chronic capsaicin (3 × 400 μg ig. for 2 weeks) in 14 healthy human subjects. The results were expressed as mean ± SEM)
9.3.14 Expression of Capsaicin Receptor, CGRP, and SP in Patients with Chronic Gastritis
9.3.14.1 Capsaicin-Sensitive Afferentation in H. pylori Positive and Negative Chronic Gastritis
The symptoms of patient’s suffering from chronic gastritis with or without H. pylori infection (20 H. pylori positive and 30 H. pylori negative) were nonspecific including epigastric discomfort, nausea, loss of appetite, and vomiting. The patients underwent physical, laboratory, ultrasonographic, endoscopic, and histological (including special immunohistochemical) examinations. Twenty people with functional dyspepsia (all of them underwent the aforementioned medical, laboratory, iconographic, and histological examinations and all of these examinations indicated negative results) were taken as healthy controls. The age of patients was 39–68 years; there were 22 males and 29 females with chronic gastritis, and 10 males and 10 females in the functional dyspepsia group.
The H. pylori infection was detected by 14C-urea breath test, rapid urease test, Warthinn-Starry silver staining, and specific histological examinations. The gastric tissue samples from the stomach and antrum were examined by an independent histologist and classified of chronic gastritis according to the Sydney System.
The immunohistochemical studies were carried out on formalin fixed, paraffin-embedded tissue samples of gastric mucosa using the peroxidase-labeled polymer method (Lab Vision Co., Fremont, USA). SP was detected by the NC1/34HL rat monoclonal antibody, the TRPV1 receptor, and CGRP were labeled using polyclonal rabbit antisera (all from Alcam Ltd., UK, Cambridge).
9.3.14.2 Capsaicin-Sensitive Afferentation in H. pylori Positive Chronic Gastritis Before and After Eradication Treatment
These observations were carried out in 38 persons, including 20 healthy subjects and 18 patients with H. pylori positive gastritis. The age of persons with histologically intact gastric mucosa (controls) were 41 to 67 years (mean = 52.2 years). The age of patients with chronic H. pylori positive gastritis (6 males, 12 females) was 39–68 years (mean = 56.4 years).
The time period between the first and control gastroscopy was 6 weeks after the starting of the examinations. The biopsies were taken from the corpus and antrum of patients with chronic gastritis, before and after eradication treatment and from healthy subjects.
The H. pylori positive patients underwent 7 days eradication treatment with a combination of double dose PPI (pantoprazole 2 × 40 mg/day), amoxicillin (1000 mg twice daily), and clarithromycin (500 mg twice daily) according to the European guidelines. After this 1 week combination treatment, the patients continued to take normal dose of PPI for another week.
The H. pylori infection was detected before and after treatment with 14C-urea breath test, rapid urease test, gastroscopy, Warthinn-Starry silver staining, and specific histological and immunohistological examinations of gastric mucosa.
The results of eradication treatment was successful in 89 %, the gastric histology (by biopsy and by histology) indicated normal picture in 22 % of cases, and 78 % of patients showed moderate gastritis.
The expression of TRPV1 and CGRP increased significantly in the gastric mucosa of patients with chronic gastritis—independently on the presence of H. pylori positive or negative status and of successful eradication treatment in patients with H. pylori positive gastritis (Fig. 9.9), meanwhile on significant expressions obtained in SP of gastric mucosa in these groups) (Dömötör et al. 2005; Lakner et al. 2011; Mózsik et al. 2011, 2014; Czimmer et al. 2013; Vincze et al. 2004) (Figs. 9.9, 9.13, 9.14 and Table 9.4).
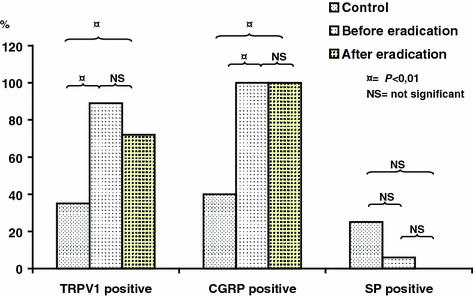
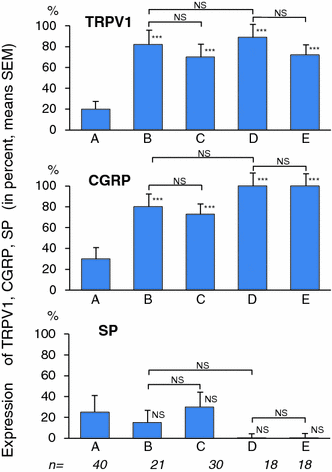

Fig. 9.13
Changes in the immunhistochemical distribution of capsaicin receptor (TRPV1), CGRP, and SP in patients with H. pylori positive chronic gastritis before and after eradication therapy (after Lakner et al. 2011)

Fig. 9.14
Changes in the expression of capsaicin receptor (TRPV1), calcitonin gene-ralated peptide (CGRP), and substance P (SP) in the human gastric mucosa of healthy voluntaries (histologically intact) (a), H. pylori positive (b), H. pylori negative (c) and H. pylori positive before, (d) and after eradication (pantoprazole 40, amoxicillin 1000 and clarithromycin 500 mg, all two times per day, for 7 days) (n = number of patients). (After Mózsik et al. 2014)
Table 9.4
The summary of the changes in the immunohistochemical distribution of capsaicin receptor, calcitonin gene-related peptide, and substance P in patient with chronic H. pylori positive gastritis before and after eradication therapy
TRPV1 | CGRP | Substance P | ||||
|---|---|---|---|---|---|---|
Positive | Negative | Positive | Negative | Positive | Negative | |
Before eradication (n = 18) | 88.89 % (16) | 11.11 % (2) | 100 % (18) | 0 % (0) | 5.56 % (1) | 94.44 % (17) |
After eradication (n = 18) | 72.22 % (13) | 17.78 % (5) | 100 % (18) | 0 % (0) | 0 % (0) | 100 % (18) |
Control group (n = 20) | 35 % (7) | 65 % (13) | 40 % (8) | 60 % (12) | 75 % (15) | 25 % (5) |
9.3.15 Clinical Pharmacological Challenges of the Natural Capsaicin for the Human Medical Treatments and Drug Processing
9.3.15.1 Chemistry of Natural Capsaicin with Respect to Spices of Capsaicin in Producing Plants in Over the World
The name of “capsaicin” is generally used in the medical physiological, pharmacological research; however, this material does not contain a clinical entity. The name of “capsaicin” covers 5 homologs (capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, homocapsaicin) and two analogs (nanonoid acid valilylamide and decanoid acid vanillylamide) (for further information, see Mózsik et al. 2009a, b).
The United States Pharmacopeia describes the list of capsaicins and their definitions, identifications, melting range, and also defines the content of capsaicin, dihydrocapsaicin, and other components as follows (USP 30-NP25, 2006, Edition pp. 1609): capsaicin contents not less than 90.0 % and not more 110.0 % of labeled percentage of total capsaicin. The content of capsaicin is not less than 55 %, and the sum of capsaicin and dihyrocapsaicin should not be less than 75.0 %. The content of other capsaicinoids cannot be more than 15 %, all these were calculated on the dried basis (see details in Ref. Mózsik et al. 2009a, b) These criteria were absolutely accepted by the Hungarian Authorities of Hungarian Institute of Pharmacy and, of course, by us during the our preclinical and clinical examinations.
9.3.15.2 International Requirements for Plant Origin Capsaicin for Orally Applicable Drug in Humans
Drug Master File (DMF)
To receive permission for human use of capsaicin preparation from the National and International Regulatory Authorities we have to show the following details: (1) Specification of capsicum species; (2) Climatic regulations of places of Capsicum cultivation; (3) Chemical treatments of Capsicum plants during their cultivation; (4) Details of treatment of Capsicum plants (their collections, drying, extractions, storages, etc.); (5) Analytical results supporting the chemical composition of the plant origin capsaicin extracts; (6) Chemical stability of natural capsaicin (capsainoids); (7) Analytical results showing the (possible) contamination of the natural capsaicin product with organic phosphates, pesticides, fusariums, aflatoxin; (8) International centification (including the Drug and Food Administration, FDA) on the capsaicin (capsaicinoids) content of the natural preparation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


