Cancers of the Pleura
Cancers often metastasize to the pleura, particularly metastatic carcinomas, but also metastatic sarcomas, lymphomas, and other types of cancer. Some cancers, particularly lung carcinomas, may also invade the pleura by direct extension. Diffuse malignant mesothelioma is the most common primary tumor of the pleura, but it is relatively rare compared to metastatic cancers. Other primary neoplasms of the pleura are very rare.
Part 1 Diffuse Malignant Mesothelioma
Alvaro C. Laga
Timothy C. Allen
Carlos Bedrossian
Bruno Murer
Philip T. Cagle
Diffuse malignant mesothelioma (DMM) of the pleura is a relatively rare tumor most commonly observed in adult males over the age of 50. The majority of DMMs are related to asbestos exposure and develop several decades after initial exposure. There is often a history of pleuritic pain and shortness of breath. Pleural effusions often occur and may be bloody and voluminous. Grossly, DMMs grow on the pleural surfaces initially as masses or multiple nodules that eventually circumferentially encase the lung in a rind-like fashion. DMMs can invade the underlying lung tissue or underlying chest wall or spread as metastases. DMM is a very aggressive tumor; traditionally, almost all patients die of the disease within 2 years of diagnosis. DMMs have three basic histologic types: epithelial, sarcomatous, and mixed or biphasic. Variations of these basic histologic types can be observed. The pathologist should be aware of the many patterns that DMM can present for diagnostic recognition, although most of them do not have any clinical significance.
Clinically, radiographically, and pathologically, many other neoplasms with pleural extension or pleural metastases can mimic DMM. Adenocarcinomas particularly mimic epithelial mesotheliomas, and spindle cell neoplasms can mimic sarcomatous mesotheliomas. A multimodal approach including conventional histology and immunohistochemistry provides the most reliable differentiation of DMM from other types of cancer. Electron microscopy may be useful particularly for epithelial mesotheliomas. It can be difficult to differentiate reactive mesothelial hyperplasia from epithelial mesothelioma and organizing pleuritis from sarcomatous mesothelioma. Demonstration of invasion into subpleural tissue is the most reliable criterion for diagnosis of DMM versus benign reactive processes. Keratin immunostain may be useful for confirming invasion into underlying tissues.
Cytologic Features
Epithelial DMMs tend to shed cells in groups or clusters, including three-dimensional morulae.
Nuclei of epithelial DMM may be round or oval and have coarse, irregular chromatin.
Cytoplasm of epithelial DMM may be vacuolated.
Epithelial DMMs are often cytologically bland but are recognized as a separate cell population within a fluid.
Occasionally, epithelial DMMs exhibit gland formation, psammoma bodies, or bizarre multinucleated cells.
Diagnosis of epithelial DMMs by cytology may be very difficult because of the bland cytologic features of many epithelial DMMs versus the worrisome cytologic atypia that can occur with reactive mesothelial cell hyperplasia.
Sarcomatous DMMs do not shed cells into the pleural fluid but may be seen as clusters of sarcomalike spindle cells on aspiration cytology.
Histologic Features
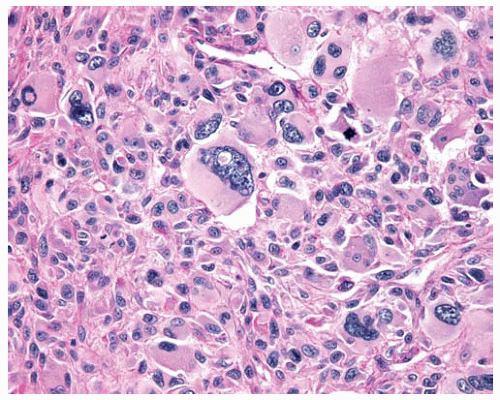
Epithelial DMMs often consist of a proliferation of round to polygonal tumor cells with abundant densely eosinophilic cytoplasm.
Nuclei of epithelial DMMs may be bland; there may be few or no mitoses.
Myxoid stroma containing hyaluronic acid may be present, especially with better differentiated epithelial DMMs.
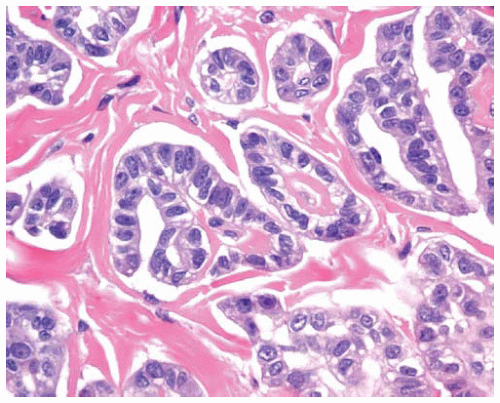
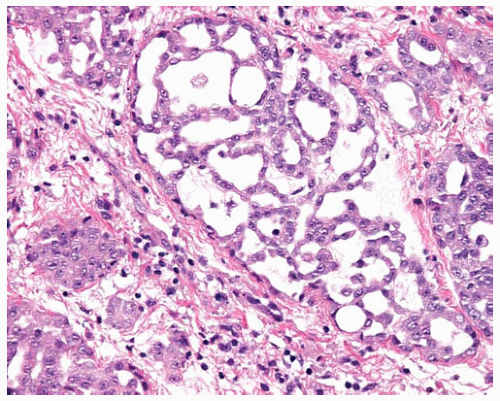
The tumor cells in epithelial DMMs may grow in one or more patterns, including solid sheets of cells or tubulopapillary patterns.
Papillae with fibrovascular cores lined by cuboidal DMM cells may be a component of epithelial DMMs; these areas should be distinguished from well-differentiated papillary mesothelioma, which has different biologic behavior (see Part 3).
Epithelial DMM may have a microglandular pattern with proliferation of small glandlike or tubular structures lined by cuboidal cells.
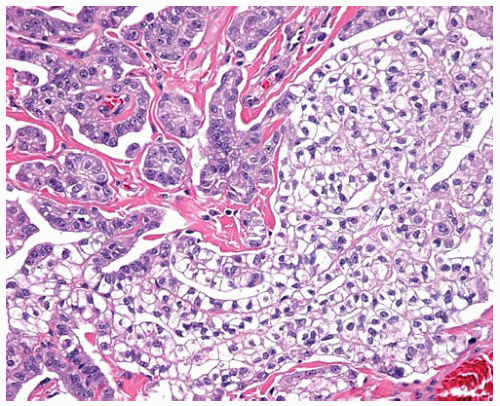
Rare patterns of epithelial DMM include anaplastic or pleomorphic patterns, clear-cell pattern, small-cell pattern, deciduoid pattern, lymphohistiocytoid pattern, and adenomatoid pattern.
Sarcomatous DMM is composed of fascicles of spindle cells with oval nuclei, amphophilic cytoplasm, and occasionally prominent nucleoli.
In general, sarcomatous DMMs show more cytologic atypia than epithelial DMMs and often show mitotic activity and necrosis.
Bland necrosis is characteristic of sarcomatous DMM and is a “clean” ischemic necrosis without basophilic cellular debris and neutrophils.
Mixed (biphasic) DMM has a combination of both epithelial areas and sarcomatous areas in the same tumor (at least 10% of each component).
Transitional DMM refers to a histopathologic pattern in which the individual cells have a transitional appearance blending features of epithelial and sarcomatous mesothelioma in the same cell.
Desmoplastic DMMs have areas with few cells in abundant dense collagen, often in plaque-like basket-weave pattern; desmoplastic DMMs are most often sarcomatous but occasionally can be epithelial or mixed; the cells may be cytologically bland or inconspicuous and are best demonstrated with keratin immunostain.
Epithelial DMMs are generally immunopositive for pan-keratin, calretinin, WT1, thrombomodulin, and HBME-1 and are generally immunonegative for adenocarcinoma markers such as MOC-31, B72.3, CEA, LeuM1, and BerEP4 (see Chapter 16).
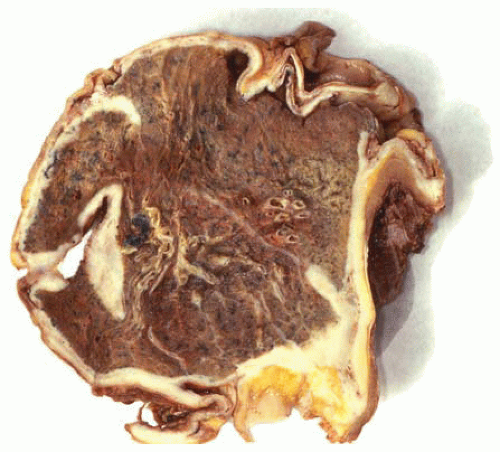 Figure 15.1 Cross-section of lung similar to CT scan image shows circumferential encasement of pleura by DMM in a rind-like fashion. |
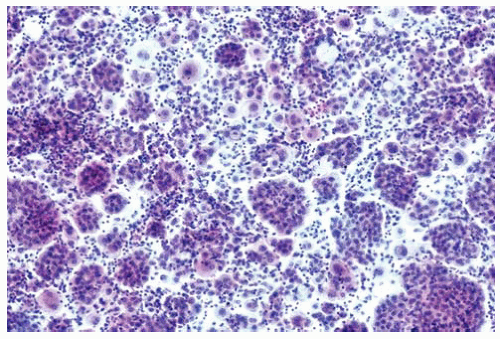 Figure 15.2 Pleural fluid cytology contains individual mesothelioma cells and three-dimensional morulae of mesothelioma cells. |
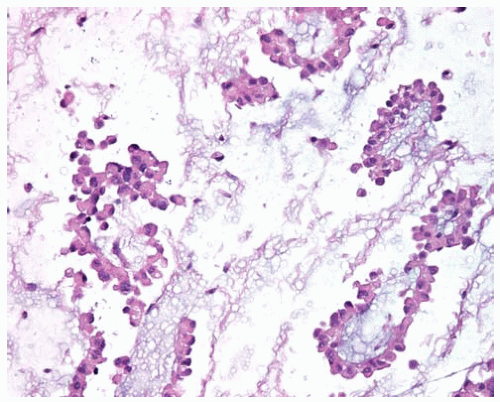 Figure 15.3 Pleural fluid has strips and clusters of bland epithelial DMM cells floating in pools of hyaluronic acid. |
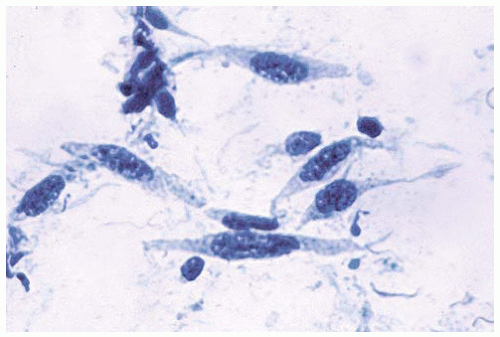 Figure 15.4 Aspiration cytology of sarcomatous DMM shows malignant spindle cells with elongated oval nuclei. |
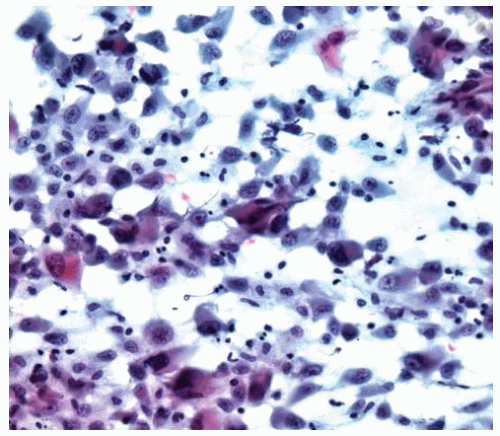 Figure 15.5 Cytology of transitional DMM shows a blend of epithelial and sarcomatous features in the same cell. |
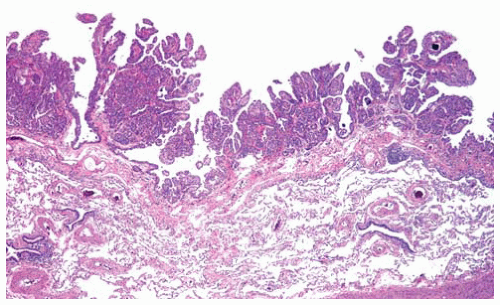 Figure 15.6 Papillary fronds of early-stage tubulopapillary DMM on the visceral pleural surface shows minimal superficial invasion into the underlying pleura; this is not a reactive hyperplasia. |
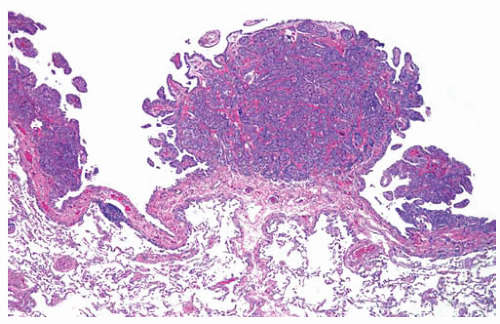 Figure 15.7 Tumor nodules of early-stage tubulopapillary DMM are observed on the visceral pleural surface; this is not a reactive hyperplasia. |
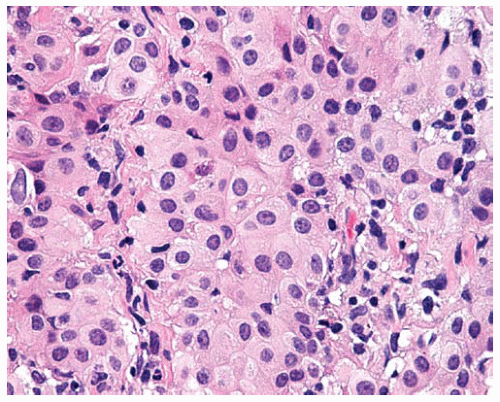 Figure 15.8 Sheets of cytologically bland epithelial DMM cells have regular, oval, eccentric nuclei, abundant cytoplasm, and distinct cell borders. |
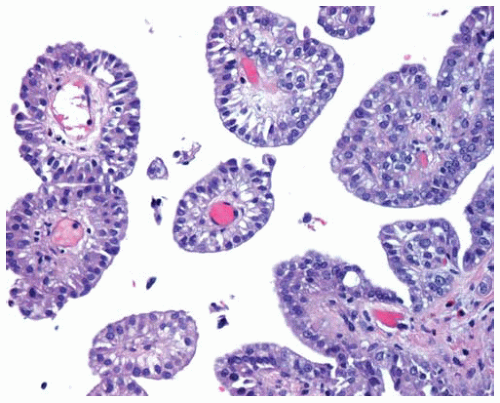 Figure 15.9 Fibrovascular cores are lined by epithelial DMM cells in a papillary DMM; biopsies limited to such areas should not be mistaken for well-differentiated papillary mesothelioma (see Part 3). |
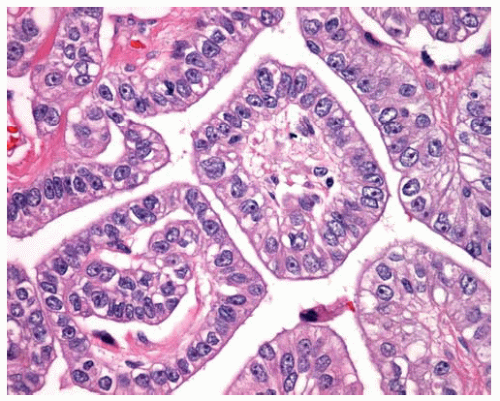 Figure 15.10 High power of fibrovascular cores lined by cuboidal malignant epithelial cells in papillary DMM. |
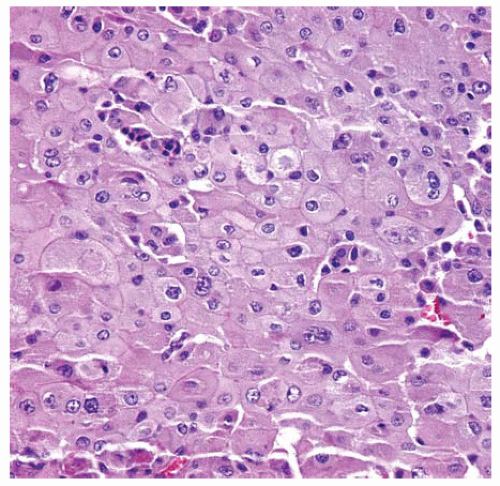 Figure 15.13 Deciduoid DMM consists of epithelial cells with plump pink cytoplasm and sharp cell borders resembling decidual cells in pregnancy. |
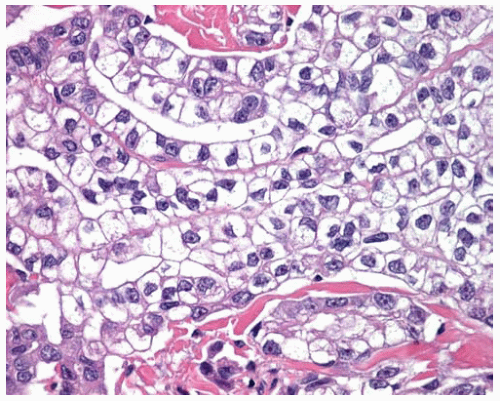 Figure 15.15 Higher power of clear cells from Figure 15.14 shows DMM cells with clear cytoplasm arranged in cords and tubular structures. |
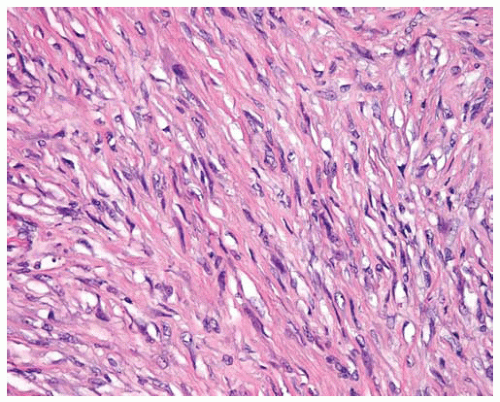 Figure 15.16 Sarcomatous DMMs are composed of malignant spindle cells and resemble sarcomas on hematoxylin and eosin (H&E)-stained sections. |
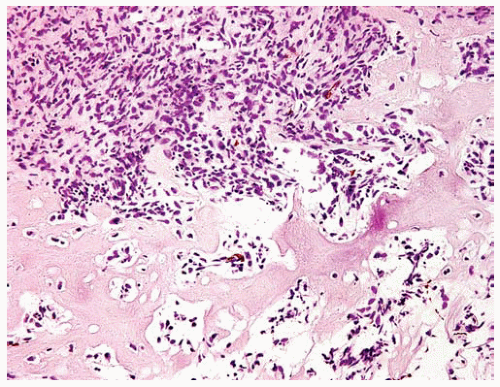 Figure 15.17 Sarcomatous DMM may have heterologous elements such as the malignant osteoid in this sarcomatous DMM. |
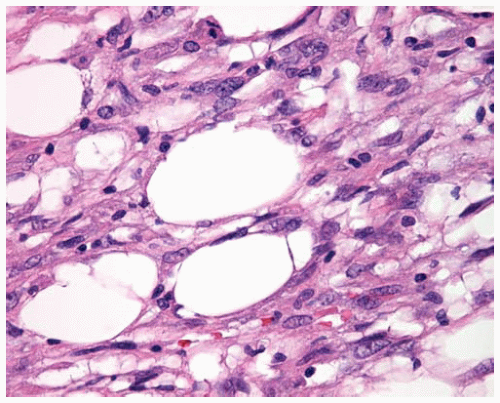
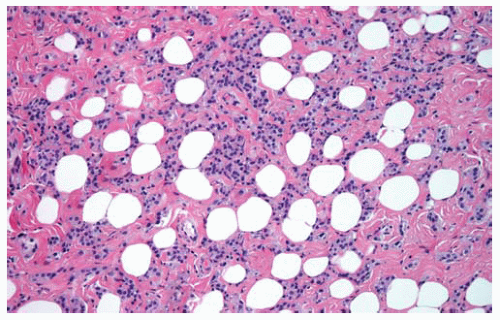 Figure 15.18 Invasion of DMM into underlying fat produces an appearance of holes (residual fat cells) resembling Swiss cheese. |
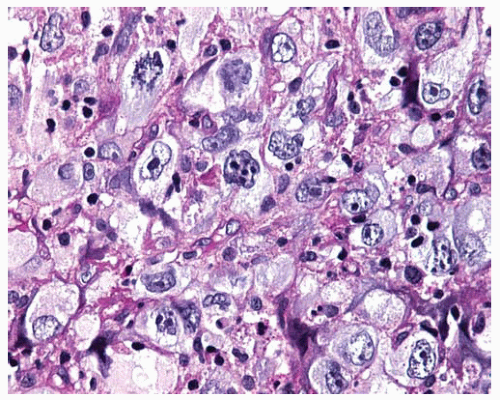 Figure 15.20 Pleomorphic DMM composed of anaplastic polygonal cells with pleomorphic nuclei, large and multiple nucleoli, and mitosis. |
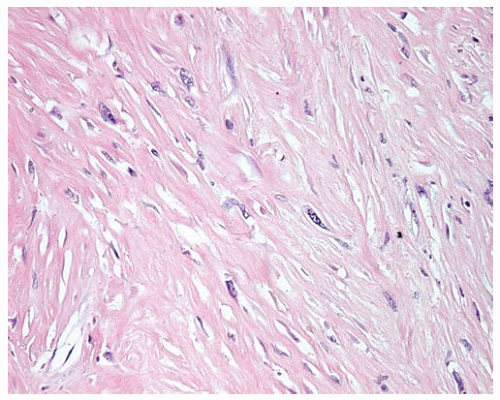 Figure 15.22 Desmoplastic DMM shows a few atypical spindle cells of sarcomatous DMM in a dense collagenized stroma. |
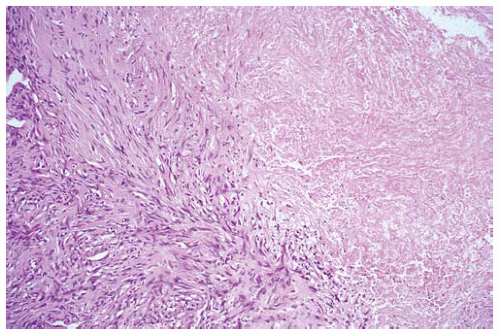 Figure 15.23 Bland necrosis in a sarcomatous DMM is “clean” ischemic necrosis lacking basophilic cellular debris and neutrophils. |
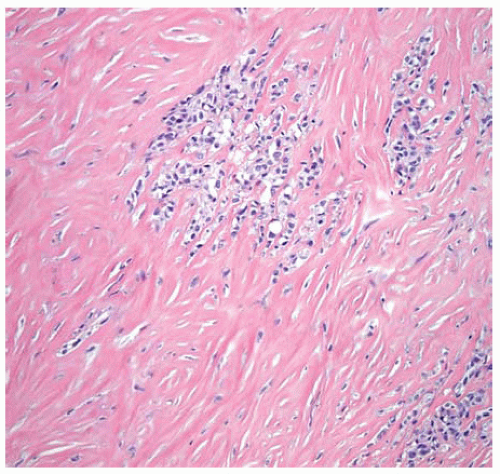 Figure 15.24 Desmoplastic DMM with a few clusters of epithelial cells in a dense collagenized stroma. |
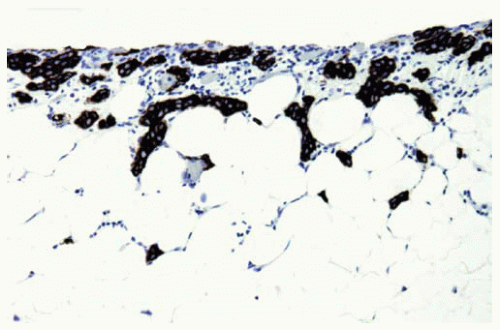 Figure 15.25 Keratin stain highlights mesothelioma cells superficially invading into adipose tissue from the pleural surface, confirming that the cells are malignant, in a very early mesothelioma. |
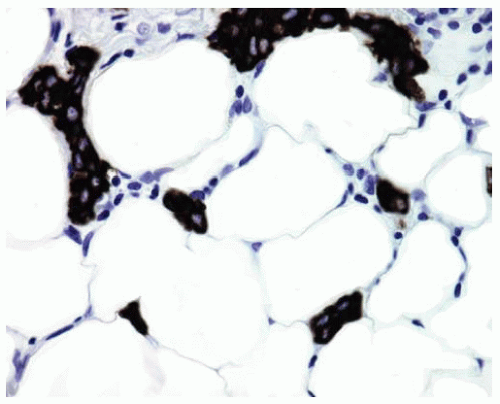 Figure 15.26 Higher power shows keratin-positive DMM cells among adipose tissue in early superficial invasion. |
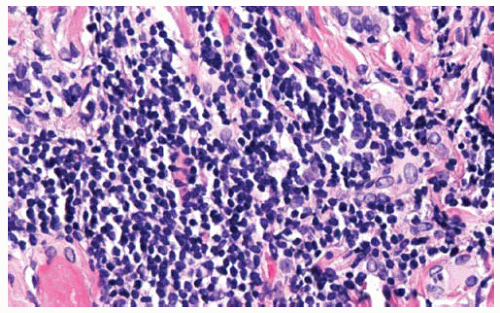 Figure 15.27 Rarely DMM cells may be obscured by intense lymphocytic infiltrates, as in this example; chronic inflammation by itself does not confirm a reactive process and exclude malignancy; on occasion DMM with intense inflammatory infiltrates may resemble lymphoma on H&E stain; keratin immunostains may assist in identifying the mesothelioma cells.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|