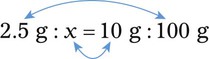
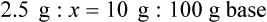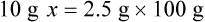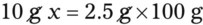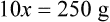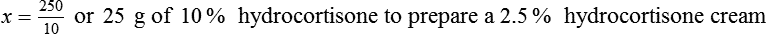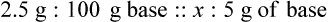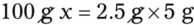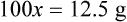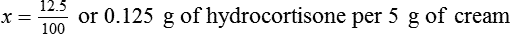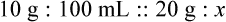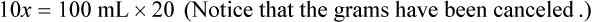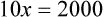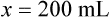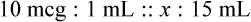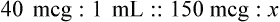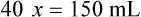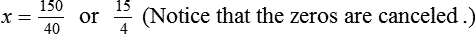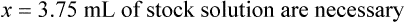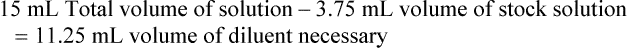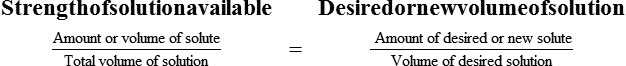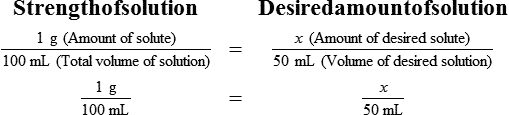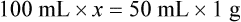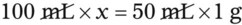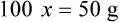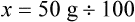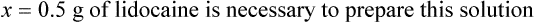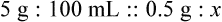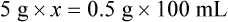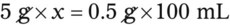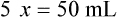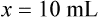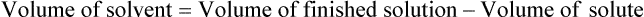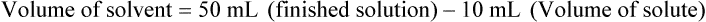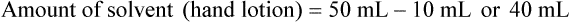Chapter 13 • Interpret labels for the weight/volume of solute in solvent • Dilute stock medications to the required strength using strengths expressed as percentages, fractions, and ratios • Calculate the weight/volume of active ingredient in a substance • Using alligation, calculate the weight/volume of stock medications needed to prepare a desired compound With percentage preparations, the easiest method for calculation is the ratio and proportion method. If a review of the ratio and proportion method is necessary, see Chapter 2. When a volume of a known solution requires reducing or enlarging to meet a physician’s order, the original strength of the preparation may be used for the calculation of the new volume or weight of the preparation. For example, using the above calculations in Example 13-4, if the physician desires 100 mL, the answer can be figured using ratio and proportion.
Calculation of Mixtures from Stock Medications
Interpreting Solution Labels and Calculating Solutions In Percentages
Reducing and Enlarging Ordered Preparations
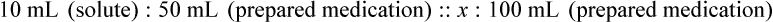
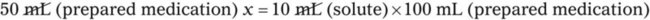
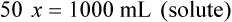
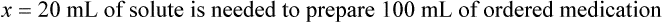
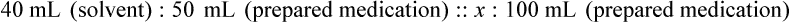
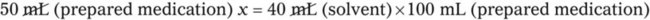
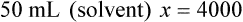
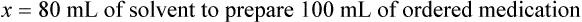
Calculation of Mixtures from Stock Medications