Calcium, Phosphate and Magnesium
Disorders of calcium metabolism are common in clinical practice and may result in hypocalcaemia or hypercalcaemia as well as bone abnormalities. Intimately associated with calcium disorders are disorders involving phosphate and magnesium metabolism.
CALCIUM METABOLISM
TOTAL BODY CALCIUM
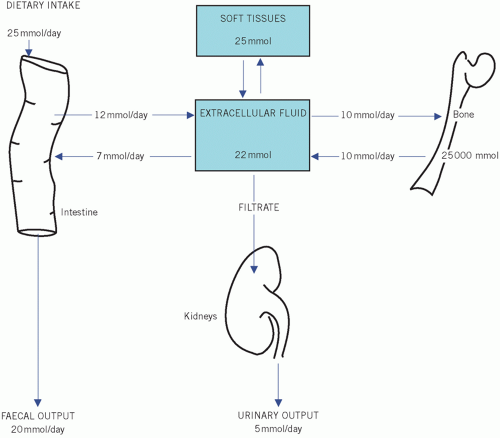
The total body calcium depends upon the calcium absorbed from dietary intake and that lost from the body (Fig. 6.1). Ninety-eight per cent of body calcium is found in the skeleton. The extraosseous fraction, although amounting to only 1 per cent of the total, is essential because of its effect on neuromuscular excitability and cardiac muscle. An important mediator of intracellular calcium is calmodulin, a calciumbinding regulatory protein.
Factors affecting calcium intake
About 25 mmol (1 g) of calcium is ingested per day, of which there is a net absorption of 6-12 mmol (0.25-0.5 g). The active metabolite of vitamin D, 1,25-dihydroxycholecalciferol (1,25-(OH)2D3, also called calcitriol), is needed for calcium absorption.
Factors affecting calcium loss
Calcium is lost in urine and faeces. Urinary calcium excretion depends on the amount of calcium reaching the glomeruli, the glomerular filtration rate (GFR) and renal tubular function. Parathyroid hormone and 1,25-dihydroxyvitamin D increase urinary calcium reabsorption.
Faecal calcium is derived from the diet and that portion of the large amount of intestinal secretions that has not been reabsorbed. Calcium in the intestine may form insoluble, poorly absorbed complexes with oxalate, phosphate or fatty acids. An excess of fatty acids in the intestinal lumen in steatorrhoea may contribute to calcium malabsorption.
CONCEPT OF PLASMA CALCIUM AND ALBUMIN CORRECTION (ADJUSTED)
The mean plasma calcium concentration in healthy subjects is tightly controlled, at around 2.15-2.55 mmol/L, and is present in two main forms:
Calcium bound to proteins, mainly albumin: this accounts for a little less than half the total calcium concentration as measured by routine analytical methods and is the physiologically inactive form.
Free ionized calcium (Ca2+), which comprises most of the rest. This is the physiologically active fraction.
Changes in plasma protein concentration, particularly of albumin, the principal plasma protein, alter the most commonly measured concentration, that of plasma total calcium, but not that of the free ionized fraction. The plasma total (but not free ionized) calcium concentration is lower in the supine than in the erect position because of the effect of posture on fluid distribution and therefore on plasma protein concentration. The direct measurement of the physiologically active free calcium ionized fraction is, for technical reasons, confined to special cases such as acid-base disturbance.
Formulae incorporating the albumin concentration have been devised in an attempt to calculate the active fraction of the plasma total calcium concentration, but, because binding is not simple, these are not always reliable, particularly if extremes of plasma albumin concentration occur. The following is a commonly used formula:

Changes in plasma hydrogen ion concentration ([H+]) affect the binding of calcium to plasma proteins because H+ competes with Ca2+ for binding sites. The plasma total calcium concentration is unaltered by changes in [H+]. If [H+] falls, as in an alkalosis, tetany may occur, despite a normal plasma total calcium concentration. Conversely, an acidosis decreases binding and so increases the proportion of plasma calcium in the free ionized form. Also, by increasing calcium solubility, it increases the rate of release of calcium from bones into the extracellular fluid (ECF). The increased load reaching the kidneys increases the renal calcium loss. Prolonged acidosis may cause osteomalacia, partly due to the buffering effect of bone.
CASE 1
A 45-year-old man was in the intensive care unit for multiple trauma following a road traffic accident. Some of his biochemistry results were as follows:
Plasma
Calcium 1.98 mmol/L (2.15-2.55)
Albumin 30 g/L (35-45)
Phosphate 0.92 mmol/L (0.80-1.35)
What is the albumin-adjusted calcium?
DISCUSSION
Adjusted calcium = 1.98 + (40 – 30) × 0.02
= 1.98 + 0.20 = 2.18 mmol/L
Note that the plasma calcium now adjusted falls within the reference range and does not require specific treatment. Remember this if the patient has hypoalbuminaemia.
Control of plasma calcium
There are a number of mechanisms by which plasma calcium concentrations are controlled. Calcium homeostasis follows the general rule that extracellular concentrations are controlled rather than the total body content. The effectiveness of this control depends upon:
an adequate supply of:
calcium,
vitamin D,
normal functioning of the:
intestine,
parathyroid glands,
kidneys.
If any one of these factors is impaired, calcium leaves bone by passive physicochemical diffusion, and plasma concentrations may be maintained at the expense of bone calcification.
Parathyroid hormone
Parathyroid hormone (PTH) is a single-chain polypeptide containing 84 residues, with its 34 N-terminal amino acids largely determining its biological activity. It is metabolized by renal, hepatic and bone cells. Renal clearance from plasma of the physiologically inert C-terminal fragment is slower than that of the N-terminal fragment, which may accumulate in plasma in renal glomerular dysfunction. The biological actions of PTH include:
stimulation of osteoclastic bone resorption, so releasing both free ionized calcium and phosphate into the ECF; this action increases the plasma concentrations of both calcium and phosphate,
decreased renal tubular reabsorption of phosphate, causing phosphaturia and increased reabsorption of calcium; this action tends to increase the plasma calcium concentration but to decrease the phosphate.
The control of PTH secretion depends on the concentration of free ionized calcium in blood circulating through the parathyroid glands. A fall increases the rate of PTH secretion, which, under physiological conditions, continues until the calcium concentration returns to normal. The secretion of PTH is also affected by the extracellular magnesium concentration, being decreased by severe, chronic hypomagnesaemia.
Detectable plasma PTH, even if the concentration is within the reference range, is inappropriate in the presence of hypercalcaemia and is consistent with primary or, more rarely, tertiary hyperparathyroidism.
Parathyroid hormone-related protein
Parathyroid hormone-related protein (PTHRP) is a peptide hormone that has a similar amino acid sequence at the biologically active end of the peptide, therefore activating the same receptors as PTH. The function of PTHRP is uncertain, but it may be important in calcium metabolism in the fetus. The gene that codes for PTHRP is widely distributed in body tissues but is normally repressed. However, it may become derepressed in certain tumours, causing humoral hypercalcaemia of malignancy.
Calcitonin
Calcitonin (produced in the C cells of the thyroid gland) decreases osteoclastic activity, slows calcium release from bone and has the opposite effect on plasma concentrations of PTH. It is probably less important than PTH in physiological homeostasis. Plasma concentrations may be very high in patients with medullary carcinoma of the thyroid, although hypocalcaemia is not usually reported in this condition. However, exogenous calcitonin has been used to treat hypercalcaemia and Paget’s disease of bone.
Metabolism and action of vitamin D
Vitamin D is derived from:
ergocalciferol (vitamin D2), obtained from plants in the diet,
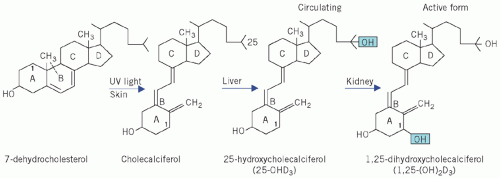
cholecalciferol (vitamin D3), formed in the skin by the action of ultraviolet light on 7-dehydrocholesterol (Fig. 6.2); this is the form found in animal tissues, especially the liver.
In normal adults, much more cholecalciferol is derived from the action of sunlight on skin (wavelength 270-310 nm) than from food. Dietary sources are important when requirements are high, such as during growth or pregnancy, or in those elderly or chronically sick individuals who are confined indoors and not exposed to the sun.
Vitamin D is transported in plasma bound to specific carrier proteins. It is inactive until metabolized. In the liver, cholecalciferol is hydroxylated to 25-hydroxycholecalciferol (25-OHD3) by the enzyme 25-hydroxylase. The rate of formation of 25-OHD3 is affected by the supply of substrate in the form of calciferol, whether derived from the skin or from the
diet. It is the main circulating form and store of the vitamin. Other hydroxylated metabolites are found, such as 24,25-(OH)2D3.
diet. It is the main circulating form and store of the vitamin. Other hydroxylated metabolites are found, such as 24,25-(OH)2D3.
In the proximal renal tubular cells of the kidney, 25-OHD3 undergoes a second hydroxylation, catalysed by the enzyme 1-α-hydroxylase to form the active metabolite 1,25-(OH)2D3.
The activity of 1-α-hydroxylase, and hence the production of 1,25-(OH)2D3, may be stimulated by:
a low plasma phosphate concentration,
an increase in plasma PTH concentration, possibly because of its phosphate-lowering effect.
Its activity is inhibited by:
hyperphosphataemia,
high levels of free ionized calcium.
The kidney is an endocrine organ, synthesizing and releasing the hormone 1,25-(OH)2D3; impairment of the final hydroxylation helps explain the hypocalcaemia of renal disease. This hormone increases calcium absorption by intestinal mucosal cells. In conjunction with PTH, it stimulates osteoclastic activity, releasing calcium from bone.
The action of PTH on bone is impaired in the absence of 1,25-(OH)2D3. A fall in plasma free ionized calcium concentration stimulates PTH secretion. The PTH enhances 1-α-hydroxylase activity and therefore stimulates 1,25-(OH)2D3 synthesis. The two hormones act synergistically on the osteoclasts of bone, releasing calcium into the circulation; 1,25-(OH)2D3 also increases calcium absorption from the intestinal lumen. In the short term, the homeostatic mechanisms involving the effects on bone are the more important; if hypocalcaemia is prolonged, more efficient absorption becomes important. Once the plasma free ionized calcium concentration is adjusted, the secretion of both PTH and 1,25-(OH)2D3 is suppressed.
Thus, 25-OHD3 is the circulating, inactive form of vitamin D and plasma concentrations fall in deficiency states. The measurement of the biologically active metabolite, 1,25-(OH)2D3, which circulates in plasma bound to vitamin D-binding protein (VDBP) in very low concentrations, is rarely indicated unless a defect in the vitamin metabolic pathway is suspected, as it does not reflect body stores.
The vitamin D receptor (VDR) is found in almost all cell nuclei with various effector systems such as endocrine, paracrine or autocrine. Calcitriol activates the receptor, which forms a heterodimer with the retinoid-X receptor and binds to hormone response elements on deoxyribonucleic acid (DNA) and is involved in the expression of various gene products. These pathways not only involve bone metabolism but also have implications for the immune system and carcinogenesis.
Calcium-sensing receptor
The calcium-sensing receptor (CaSR) is a G proteincoupled receptor. This allows the parathyroid cells and the ascending loop of Henle epithelial cells to respond to changes in extracellular calcium. The parathyroid cell surface is rich in CaSR, which allows PTH secretion to be adjusted rapidly depending on the calcium concentration.
Defects in the CaSR gene are responsible for various rare defects of calcium homeostasis. Inactivating mutations include familial benign
hypocalciuric hypercalcaemia and neonatal severe hyperparathyroidism; activating mutations include autosomal dominant hypocalcaemia with hypercalciuria. Calcimimetic agents have been devised that bind and activate the CaSR, resulting in decreased PTH release and reduced plasma calcium concentrations.
hypocalciuric hypercalcaemia and neonatal severe hyperparathyroidism; activating mutations include autosomal dominant hypocalcaemia with hypercalciuria. Calcimimetic agents have been devised that bind and activate the CaSR, resulting in decreased PTH release and reduced plasma calcium concentrations.
Miscellaneous mechanisms of calcium control
Thyroid hormone excess may be associated with the histological appearance of osteoporosis and with increased faecal and urinary excretion of calcium, probably following its release from bone. Hypercalcaemia is a very rare complication of severe hyperthyroidism. Unless there is gross excess of thyroid hormone, the effects on plasma calcium are overridden by homeostatic reduction of PTH secretion and by urinary loss.
Other hormones influencing calcium metabolism include oestrogens, prolactin and growth hormone. These may increase 1,25-(OH)2D3 production and increase calcium absorption during pregnancy, lactation and growth.
DISORDERS OF CALCIUM METABOLISM
The consequences of most disturbances of calcium metabolism can be predicted from knowledge of the actions of PTH on bone and on renal tubular cells, and from plasma concentrations of calcium and phosphate. A low plasma free ionized calcium concentration normally stimulates PTH secretion, which results in phosphaturia; the loss of urinary phosphate over-rides the tendency to hyperphosphataemia due to the action of PTH on bone.
Consequently, the plasma phosphate concentration is usually low when the plasma PTH concentration is increased. Conversely, a high plasma free ionized calcium concentration, unless due to inappropriate excess of PTH, inhibits PTH secretion and causes a high plasma phosphate concentration. Therefore plasma calcium and phosphate concentrations usually vary in the same direction unless:
renal glomerular dysfunction is severe enough to impair the phosphaturic (and therefore hypophosphataemic) effect of PTH or PTHRP,
Hypercalcaemia
Clinical effects of an increased plasma albuminadjusted calcium concentration
Renal effects.
Renal damage is one of the most serious clinical consequences of prolonged hypercalcaemia. Because of the high plasma free ionized calcium concentration, the solubility of calcium phosphate may be exceeded and precipitate in extraosseous sites such as the kidneys (see Chapter 3).
Polyuria, characteristic of chronic hypercalcaemia, may result from impairment of renal concentrating ability owing to calcification of the tubular cells; acute hypercalcaemia may cause reversible inhibition of the tubular response to antidiuretic hormone rather than to cell damage. These effects can lead to dehydration.
Renal calculi, without significant parenchymal damage, may be caused by precipitation of calcium salts in the urine if the free ionized calcium concentration is high in the glomerular filtrate owing to hypercalcaemia (see Chapter 3).
Hypokalaemia, often with a metabolic alkalosis, is associated with hypercalcaemia. Calcium may directly inhibit potassium reabsorption from the tubular lumen (see Chapter 5).
High extracellular free ionized calcium concentrations can depress neuromuscular excitability in both voluntary and involuntary muscle. There may also be muscular hypotonia.
Depression, anorexia, nausea and vomiting, associated with high plasma calcium concentrations, are probably caused by an effect on the central nervous system.
Calcium stimulates gastrin (and therefore gastric acid) secretion. There is an association between chronic hypercalcaemia and peptic ulceration. The patient may complain of constipation and abdominal pain. Hypercalcaemia may also present as an acute abdomen.
Some patients with hypercalcaemia may be hypertensive. If renal damage is not severe, the hypertension may respond to reducing the plasma calcium concentration.
Severe hypercalcaemia causes characteristic changes in the electrocardiogram (ECG), with shortening of
the Q-T interval and broadening of the T waves. If plasma concentrations exceed about 3.5 mmol/L, there is a risk of sudden cardiac arrest or ventricular arrhythmias. For this reason severe hypercalcaemia should be treated as a matter of urgency.
Hypercalcaemia is also associated with bone and joint pain.
‘Bones, moans, groans and stones’ is a useful mnemonic to remember some of these clinical consequences of hypercalcaemia.
Causes of hypercalcaemia (Box 6.1)
Overall, thiazides are one of the most common causes of mild hypercalcaemia. However, most causes of severe hypercalcaemia are related to either primary hyperparathyroidism or malignancy. In the case of the latter, 80 per cent are due to bony metastases, with the remainder being mainly due to ectopic PTHRP. Some causes of hypercalcaemia are depicted in Box 6.1.
Box 6.1 Some causes of hypercalcaemia
Malignancy
Bony metastases, e.g. breast, lung, prostate, kidney, thyroid
Solid tumours with humoral effects
Haematological tumours, e.g. multiple myeloma
Parathyroid hormone abnormalities
Primary hyperparathyroidism (adenoma, hyperplasia, carcinoma or associated with multiple endocrine neoplasia)
Tertiary hyperparathyroidism
Lithium-induced hyperparathyroidism
High bone turnover
Thyrotoxicosis
Immobilization, e.g. with Paget’s disease
High levels of vitamin D
Vitamin D toxicity
Granulomatous disease, e.g. sarcoidosis, tuberculosis
Drugs
Thiazides (reduced renal calcium excretion)
Vitamin A toxicity
Milk-alkali syndrome
Familial hypocalciuric hypercalcaemia
Other endocrine causes
Adrenal insufficiency
Acromegaly
Rarer causes
Williams’ syndrome
Human immunodeficiency virus (HIV) infection
Leprosy
Histoplasmosis
Berylliosis
True free ionized or albumin-adjusted hypercalcaemia with hypophosphataemia is usually caused by inappropriate secretion of PTH or PTHRP. The term ‘inappropriate secretion’ is used in this book to indicate that the release of hormone into the circulation is not adequately inhibited by negative feedback control. Inappropriate PTH secretion occurs in the following clinical situations:
production of PTH by the parathyroid glands due to:
primary hyperparathyroidism,
tertiary hyperparathyroidism.
If renal glomerular function is adequate, the high circulating PTH or PTHRP concentrations cause hypercalcaemia, which is associated with a low-normal or low plasma phosphate concentration in relation to GFR, and to phosphaturia. If glomerular damage develops due to hypercalcaemia, the kidneys cannot respond normally to the phosphaturic effect of PTH and, because of impaired hydroxylation of 25-OHD3, plasma calcium concentrations may fall towards or within the reference range as renal failure progresses. Because plasma phosphate concentrations tend to rise, diagnosis may be difficult at this stage.
CASE 2
A 53-year-old man saw his general practitioner because of bone pain and constipation. A number of laboratory tests were requested, the results for the most relevant of which were as follows:
Plasma
Albumin-adjusted calcium 2.96 mmol/L (2.15-2.55)
Phosphate 0.62 mmol/L (0.80-1.35)
Parathyroid hormone 157 ng/L (20-65)
DISCUSSION
The patient has hypercalcaemia. Note also the hypophosphataemia and inappropriately raised PTH concentration. The diagnosis was subsequently found to be primary hyperparathyroidism due to a parathyroid adenoma associated with multiple endocrine neoplasia (MEN) type I. His symptoms are typical of chronic hypercalcaemia.
The clinical features of PTH- or PTHRP-induced hypercalcaemia are due to:
excess circulating concentration of free ionized calcium that is the direct consequence of increased osteoclastic activity and release of calcium from bone, and enhanced absorption of calcium from the intestinal lumen by vitamin D; PTH increases the formation of 1,25-(OH)2D3,
The differences between the clinical presentations associated with inappropriately high plasma PTH concentrations depend on the duration of the disease. The following effects on bone become evident only in long-standing cases. Prolonged decalcification of bone causes a secondary increase in osteoblastic activity. Alkaline phosphatase-rich osteoblasts release the enzyme into the circulation and, if the number of cells is greatly increased, plasma alkaline phosphatase activity rises.
Primary hyperparathyroidism
This is caused by inappropriate secretion of PTH by the parathyroid glands, causing hypercalcaemia. It is usually due to one or more parathyroid adenomas, but occasionally to hyperplasia of all four parathyroid glands or to carcinoma of one of the glands. Ectopic parathyroid tumours do also occur. Primary hyperparathyroidism may be associated with other multiple endocrine neoplasias (MENs), such as pituitary and pancreatic adenomas (MEN type I), or with phaeochromocytomas and medullary carcinoma of the thyroid (MEN type II). The incidence of primary hyperparathyroidism increases with age, being most common in elderly females.
The majority of cases of primary hyperthyroidism are diagnosed after the chance finding of high plasma calcium, usually with low plasma phosphate concentrations.
Where there are clinical symptoms and signs at presentation, these are due to hypercalcaemia and include the following:
Generalized ill health Depression, nausea, anorexia and abdominal pain and polyuria.
Renal calculi About 10 per cent of patients who present with renal calculi have primary hyperparathyroidism.
Bone pain In most patients, subperiosteal bone erosions or cysts may be seen on radiography of the terminal phalanges. However, extensive, severe bone disease, osteitis fibrosa cystica, is now a rare presenting feature, as patients are usually diagnosed before the disorder is extensive, and consequently plasma alkaline phosphatase activity is usually normal or only slightly increased. There are increased numbers of osteoclasts and an increased risk of bone fracture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree