BURNS
A severe thermal injury is one of the most devastating physical and psychological injuries a person can suffer. Over 2 million injuries due to burns require medical attention each year in the United States, with 14,000 deaths resulting. Fires in the home are responsible for only 5% of burn injuries but for 50% of burn deaths—most due to smoke inhalation. About 75,000 patients require hospitalization every year, and 25,000 of those remain hospitalized for over 2 months—evidence of the severity of illness associated with this injury.
The skin is the largest organ of the body, ranging in area from 0.25 m2 in the newborn to 1.8 m2 in the adult. It consists of two layers: the epidermis and the dermis (corium). The outermost cells of the epidermis are dead cornified cells that act as a tough protective barrier against the environment, including bacterial invasion and chemical exposure. The inner cells of the epidermis are metabolically active, producing compounds like growth factor, which help the ongoing replication process every 2 weeks. The second, thicker layer, the dermis (0.06-0.12 mm), is composed chiefly of fibrous connective tissue. The dermis contains the blood vessels and nerves to the skin and the epithelial appendages of specialized function like sweat glands. The nerve endings that mediate pain are found in the dermis.
The dermis is a barrier that prevents loss of body fluids by evaporation and loss of excess body heat. Sweat glands help maintain body temperature by controlling the amount of water that evaporates. The dermis is also interlaced with sensory nerve endings that mediate the sensations of touch, pressure, pain, heat, and cold. This is a protective mechanism that allows an individual to adapt to changes in the physical environment.
The skin produces vitamin D, which is synthesized by the action of sunlight on certain intradermal cholesterol compounds.
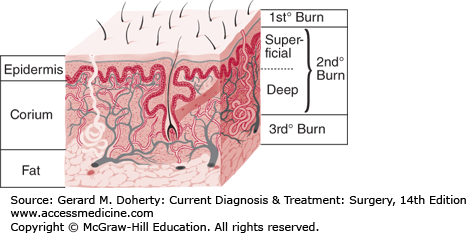
The depth of the burn (Figure 14–1) significantly affects all subsequent clinical events. The depth may be difficult to determine and in some cases is not known until after spontaneous healing has occurred or when the eschar is surgically removed or separates, exposing the wound bed.
Traditionally, burns have been classified as first-, second-, and third-degree, but the current emphasis on burn healing has led to classification as partial-thickness burns, which can heal spontaneously, and full-thickness burns, which require skin grafting, although deep partial-thickness burns are usually excised and grafted as well.
A first-degree burn involves only the epidermis and is characterized by erythema and minor microscopic changes; tissue damage is minimal, protective functions of the skin are intact, skin edema is minimal, and systemic effects are rare. Pain, the chief symptom, usually resolves in 48-72 hours, and healing takes place uneventfully. In 5-10 days, the damaged epithelium peels off in small scales, leaving no residual scarring. The most common causes of first-degree burns are overexposure to sunlight and brief scalding.
Second-degree or partial-thickness burns are deeper, involving all of the epidermis and some of the corium or dermis. The systemic severity of the burn and the quality of subsequent healing are directly related to the amount of undamaged dermis. Superficial burns are often characterized by blister formation, while deeper partial-thickness burns have a reddish appearance or a layer of whitish, nonviable dermis firmly adherent to the remaining viable tissue. Blisters, when present, continue to increase in size in the postburn period as the osmotically active particles in the blister fluid attract water. Complications from superficial second-degree burns are mainly severe pain related. These burns usually heal with minimal scarring in 10-14 days unless they become infected.
Deep dermal burns heal over a period of 4-8 weeks with only a fragile epithelial covering developing that arises from the residual uninjured epithelium of the deep dermal sweat glands and hair follicles. Severe hypertrophic scarring occurs when such an injury heals; the resulting epithelial covering is prone to blistering and breakdown. Evaporative losses after healing remain high compared with losses in normal skin. Conversion of a partial thickness injury to a full-thickness burn by the actions of wound bacteria is common. Skin grafting of deep dermal burns, when feasible, improves the biologic quality and appearance of the skin cover.
Full-thickness (third-degree) burns have a characteristic white, dry, waxy appearance and may appear to the untrained eye as unburned skin. Burns caused by prolonged exposure to heat, with involvement of fat and underlying tissue, may be brown, dark red, or black. The diagnostic findings of full-thickness burns are lack of sensation in the burned skin, lack of capillary refill, and a leathery texture that is unlike normal skin. All dermal epithelial elements are destroyed, leaving no potential for reepithelialization.
Illness and death are related to the size (surface area) and depth of the burn, the age and prior state of health of the victim, the location of the burn wound, and the severity of associated injuries, particularly lung injuries caused by smoke inhalation.
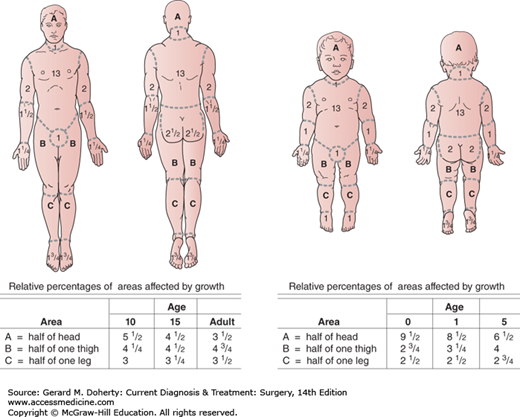
The total body surface area involved in the burn is most accurately determined by using the age-related charts designed by Lund and Browder (Figure 14–2). A set of these charts should be filled out for every burn patient on admission and when resuscitation is begun.
Figure 14–2.
Table for estimating extent of burns. In adults, a reasonable system for calculating the percentage of body surface burned is the “rule of nines”: Each arm equals 9%, the head equals 9%, the anterior and posterior trunk each equal 18%, and each leg equals 18%; the sum of these percentages is 99%.
A careful calculation of the percentage of total body burn is useful for several reasons. First, there is a general clinical tendency to both underestimate and overestimate the size of the burn and thus its severity. The American Burn Association has adopted a severity index for burn injury (Table 14–1). Second, prognosis is directly related to the extent of injury both size and depth. Third, the decision about who should be treated in a specialized burn facility or managed as an outpatient is based on the estimate of burn size and depth.
| Major Burn Injury |
| Second-degree burn of > 25% body surface area in adults |
| Second-degree burn of > 20% body surface area in children |
| Third-degree burn of > 10% body surface area |
| Most burns involving hands, face, eyes, ears, feet, or perineum |
Most patients with the following:
|
| Moderate Uncomplicated Burn Injury |
| Second-degree burn of 15%-25% body surface area in adults |
| Second-degree burn of 10%-20% body surface area in children |
| Third-degree burn of < 10% body surface area |
| Minor Burn Injury |
| Second-degree burn of < 15% body surface area in adults |
| Second-degree burn of < 10% body surface area in children |
| Third-degree burn of < 2% body surface area |
Patients under age 2 years and over age 60 years have a significantly higher death rate for any given extent of burn. The higher death rate in infants results from a number of factors. First, the body surface area in children relative to body weight is much greater than in adults. Therefore, a burn of comparable surface area has a greater physiologic impact on a child. Second, immature kidneys and liver do not allow for removal of a high solute load from injured tissue or the rapid restoration of adequate nutritional support. Third, the incompletely developed immune system increases susceptibility to infection. Associated conditions such as cardiac disease, diabetes, or chronic obstructive pulmonary disease significantly worsen the prognosis in elderly patients.
Burns involving the hands, face, feet, or perineum are at risk for severe complications if not properly treated. Patients with such burns should always be admitted to the hospital, preferably to a burn center. Chemical and electrical burns or those involving the respiratory tract are invariably far more extensive than is evident on initial inspection. Therefore, hospital admission is also necessary in these cases.
The microscopic pathologic feature of the burn wound is principally surface coagulation necrosis. Burned tissue has three distinct zones. The first is the zone of “coagulation,” or necrosis with irreversible cell death and no capillary blood flow. Surrounding this is a zone of injury or stasis, characterized by sluggish capillary blood flow and injured cells. Although damaged, the tissue is still viable. Further tissue injury can be caused by products of inflammation such as oxidants and vasoconstrictor mediators. Environmental insults such as hypoperfusion, desiccation, or infection can also cause the injured tissue to become necrotic. This process is called wound conversion. The third zone is that of “hyperemia,” which is the usual inflammatory response of healthy tissue to nonlethal injury. Vasodilatation and increased capillary permeability is typically present.
A rapid loss of intravascular fluid and protein occurs through the heat-injured capillaries. The volume loss is greatest in the first 6-8 hours, with capillary integrity returning toward normal by 36-48 hours. A transient increase in vascular permeability also occurs in nonburned tissues, probably as a result of the initial release of vasoactive mediators. However, the edema that develops in nonburned tissues during resuscitation appears to be due in large part to the marked hypoproteinemia caused by protein loss into the burn itself. A systemic inflammatory response occurs in response to a large body burn, resulting in the release of oxidants and other inflammatory mediators into unburned tissues. A generalized decrease in cell energy and membrane potential occurs as a result. This leads to a shift of extracellular sodium and water into the intracellular space. This process is also corrected as hemodynamic stability is restored but will return if the systemic inflammation is amplified. Smoke inhalation markedly increases the hemodynamic instability, fluid requirements, and mortality rates by adding another source of intense inflammation leading to local lung and systemic tissue damage.
The initial metabolic response appears to be activated by proinflammatory cytokines and in turn oxidants. The secretion of catecholamines, cortisol, glucagon, renin-angiotensin, antidiuretic hormone, and aldosterone is also increased. Early in the response, energy is supplied by the breakdown of stored glycogen and by the process of anaerobic glycolysis.
Profound hypermetabolism and catabolism occur in the postburn period, characterized by an increase in metabolic rate that approaches doubling of the basal rate in severe burns and a rapid loss of the components of lean body mass with muscle loss exceeding a pound a day. The degree of response is proportionate to the degree of injury, with a plateau occurring when the burn involves about 70% of the total body surface. The initiating and perpetuating factors are the mediators of inflammation, especially the cytokines and endotoxin. Added environmental stresses such as pain, cooling, and sepsis syndrome increase the obligatory hypermetabolism and catabolism.
During the first postburn week, the metabolic rate (or heat production) and oxygen consumption rise progressively from the normal level present during resuscitation and remain elevated until the wound is covered and no other sources of inflammation remain. The specific pathophysiologic mechanism remains undefined, but increased and persistent catecholamine and cortisol secretion are major factors, as is increased circulating endotoxin absorbed from wound or gut and proinflammatory cytokines.
The evaporative water loss from the wound may reach 300 mL/m2/h (normal is about 15 mL/m2/h). This produces a heat loss of about 580 kcal/L of water evaporated. Covering the burn with an impermeable membrane, such as skin substitute, reduces the heat loss. Similarly, placing the burn patient in a warm environment, where convection and radiant loss of heat are minimized, also modestly reduces the heat loss and the metabolic rate. The persistently elevated circulating levels of catecholamines and cortisol stimulate an exaggerated degree of gluconeogenesis and protein breakdown. Protein catabolism, glucose intolerance, and marked total body weight loss result.
Aggressive nutritional support along with rapid wound closure and control of pain, stress, and sepsis will help control the hypermetabolic catabolic state. Controlled use of a beta-blocker has been shown to decrease catabolism. In addition, insulin, growth hormones, and testosterone analogues have been shown to both decrease catabolism and increase anabolism.
A number of immunologic abnormalities in burn patients predispose to infection. Serum IgA, IgM, and IgG are frequently depressed, reflecting depressed B-cell function. Cell-mediated immunity or T-cell function is also impaired, as demonstrated by prolonged survival of homografts and xenografts.
Polymorphonuclear (PMN) chemotactic activity is suppressed. A decrease in chemotaxis predates evidence of clinical sepsis by several days. Decreased oxygen consumption and impaired bacterial killing have also been demonstrated in PMNs. Depressed killing is probably due to decreased production of hydrogen peroxide and superoxide; this has been demonstrated by decreased PMN chemiluminescent activity in burn patients.
BURN MANAGEMENT
The burn patient should be assessed and treated like any patient with major trauma. The first priority is to ensure an adequate airway. If there is a possibility that smoke inhalation has occurred—as suggested by exposure to a fire in an enclosed space or burns of the face, nares, or upper torso—arterial blood gases and arterial oxygen saturation of hemoglobin and carboxyhemoglobin CoHgb levels should be measured, and 100% oxygen should be administered. If CoHgb is elevated, 100% oxygen should be administered until levels return to normal.
Endotracheal intubation is indicated if the patient is semicomatose, has deep burns to the face and neck, or is otherwise critically injured. Intubation should be done early in all doubtful cases, because delayed intubation will be difficult to achieve in cases associated with facial and pharyngeal edema or upper airway injury, and an emergency tracheostomy may become necessary later under difficult circumstances. Respiratory support is necessary for severe smoke damage to the lower airways. If the burn exceeds 20% of body surface area, a urinary catheter should be inserted to monitor urine output. A large-bore intravenous catheter should be inserted, preferably into a large peripheral vein. There is a significant complication rate with the use of central lines in burn patients owing to the increased risk of infection.
Severe burns are characterized by large losses of intravascular fluid, which are greatest during the first 8-12 hours. Fluid loss occurs as a result of the altered capillary permeability, severe hypoproteinemia, and the shift of sodium into the cells. Both fluid shifts diminish significantly by 24 hours postburn. The lung appears to be reasonably well protected from the early edema process, and pulmonary edema is uncommon during the resuscitation period unless there is a superimposed inhalation injury. Increasing perfusion rather than infusion of bicarbonates is the appropriate approach.
Initially, an isotonic crystalloid salt solution is infused to counterbalance the loss of plasma volume into the extravascular space and the further loss of extracellular fluid into the intracellular space. Lactated Ringer solution is commonly used, the rate being dictated by urine output, pulse (character and rate), state of consciousness, and, to a lesser extent, blood pressure. Urine output should be maintained at 0.5 mL/kg/h and the pulse at 120 beats/min or slower. Base deficit has been shown to be an excellent marker, with an increasing deficit indicating inadequate perfusion.
Swan-Ganz catheters and central venous pressure lines are seldom needed except in the case of severe smoke inhalation injury or unless the patient has sufficient cardiopulmonary disease that accurate monitoring of volume status would be difficult without measurement of filling pressures or unless a persistent base deficit is present, indicating continued impaired perfusion. The amount of lactated Ringer necessary in the first 24 hours for adequate resuscitation is approximately 3-4 mL/kg of body weight per percent of body burn, which is the amount of fluid needed to restore the estimated sodium deficit. At least half of the fluid is given in the first 8 hours because of the greater initial volume loss. Dextrose-containing solutions are not used initially because of early stress-induced glucose intolerance.
Although the importance of restoring colloid osmotic pressure and plasma proteins is well recognized, the timing of colloid infusion remains somewhat varied. Plasma proteins are ordinarily not infused until after the initial plasma leak begins to decrease. This usually occurs about 4-8 hours postburn. The addition of a protein infusion to the treatment regimen after this period will decrease the fluid requirements and—in very young or elderly patients and in patients with massive burns (in excess of 50% of body surface)—will improve hemodynamic stability.
After intravenous fluids are started and vital signs stabilized, the wound should be debrided of all loose skin and dirt. To avoid severe hypothermia, debridement is best done by completing one body area before exposing a second. An alternative is to use an overhead radiant heater, which will decrease net heat loss. Cool water is a very good analgesic on a small superficial burn; however, it should not be used for larger burns because of the risk of hypothermia. Pain is best controlled with the use of intravenous rather than intramuscular narcotics. Tetanus toxoid, 0.5 mL, should be administered to patients with any significant burn injury.
Treatment should aim to decrease excessive catecholamine stimulation and provide enough calories to offset the effects of the hypermetabolism. Hypothermia, pain, and anxiety all need to be aggressively controlled. Hypovolemia should be prevented by giving enough fluid to make up for the body losses.
Ongoing management of any smoke inhalation injury will be necessary using vigorous pulmonary toilet to avoid airway plugging and hypoxia. Nutritional support should begin as early as possible in the postburn period to maximize wound healing and minimize immune deficiency. Patients with moderate body burns may be able to meet nutritional needs by voluntary oral intake. Patients with large burns invariably require calorie and protein supplementation to reach a goal of 30 cal/kg body weight for calories and 1.5 g/kg body weight for protein. This can usually be accomplished by administering a formula diet through a small feeding tube. Parenteral nutrition is also occasionally required, but the intestinal route is preferred if needs can be met this way. Early restoration of gut function will also decrease gut bacterial translocation and endotoxin leak.
Vitamins A, E, and C and zinc should be given until the burn wound is closed. Low-dose heparin therapy may be beneficial, as with other immobilized patients with soft tissue injury.
In the management of superficial partial or second-degree burns, one must provide as aseptic an environment as possible to prevent infection. However, superficial burns generally do not require the use of topical antibiotics. Occlusive dressings are used to minimize exposure to air, increase the rate of reepithelialization, and decrease pain. The exception is the face, which can be treated open with an antibacterial ointment. If there is no infection, burns will heal spontaneously.
The goals in managing deep partial-thickness or full-thickness (third-degree) burns are to prevent invasive infection (ie, burn wound sepsis), to remove dead tissue, and to cover the wound with skin or skin substitutes as soon as possible.
All topical antibiotics retard wound healing to some degree and therefore should be used only on deep second- or third-degree burns or wounds, which have a high risk of infection.
Topical agents have definitely advanced the care of burn patients. Although burn wound sepsis is still a major problem, the incidence is lower and the death rate has been markedly reduced, particularly in burns of less than 50% of body surface area. A silver-containing product is the treatment of choice because silver has superior antimicrobial properties. Silver sulfadiazine is the most widely used preparation. Mafenide, silver nitrate, povidone-iodine, and gentamicin ointments are also used. Silver release dressings are now very popular. A secondary dressing is placed over the silver release dressing to retain heat and optimize the wound environment.
Silver sulfadiazine, a cream that is effective against a wide spectrum of gram-positive and gram-negative organisms, is only moderately effective in penetrating the burn eschar. A transient leukopenia secondary to bone marrow suppression often occurs with the use of silver sulfadiazine in large burns, but the process is usually self-limiting, and the agent does not have to be discontinued.
Silver release dressings are available in a slow-release form that release silver ions for several days, decreasing dressing changes and improving patient comfort.