Elizabeth B. Pearsall
Breast Surgery
Most surgical procedures of the breast are performed to establish a definitive diagnosis or to treat breast cancer. Changing hormone levels from puberty throughout the remainder of life affect breast tissue in its physical and microscopic characteristics. In association with these changes, numerous aberrations and tumors can occur.
The occurrence of breast changes, benign or malignant, are some of the most emotionally upsetting health problems confronting women. Besides skin cancer, breast cancer is the most common cancer in American women. The most significant risk factors for developing breast cancer are being a woman and growing older (Breast Cancer.org 2012). Estimates are that one in eight women in the United States will develop breast cancer during her life. If the cancer is detected early, there is a 98.5% 5-year survival rate (Surveillance Epidemiology and End Results, 2013). Breast cancer risk doubles if a woman’s mother, sister, or daughter had breast cancer. Early menarche (before 12 years of age) and a late natural menopause (after 50 years of age) are associated with a slightly increased risk for developing breast cancer. Further, a woman who has cancer in one breast is at increased risk for cancer in the other breast (American Cancer Society [ACS], 2012). Heightened public awareness, an increased number of women practicing self-examination, early detection of breast masses by mammography, and reduced use of hormone replacement therapy following the results of a large study called the Women’s Health Initiative have started to slow the annual increase in breast cancer mortality.
Surgical Anatomy
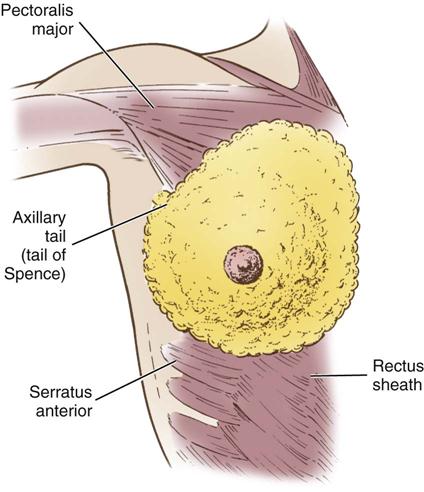
The breasts are bilateral mammary glands that lie on the pectoralis major fascia of the anterior chest wall. They are surrounded by a layer of fat and are encased in an envelope of skin. The breasts extend from the second to the sixth ribs and horizontally from the lateral edge of the sternum to the anterior axillary line. The largest part of the mammary gland rests on the connective tissue of the pectoralis major muscle and laterally on the serratus anterior (upper outer quadrant of the breast), with a normal globular contour occurring as a result of fascia support (Cooper ligaments) (Figure 17-1). An elongation of mammary tissue normally extends laterally on the pectoralis major toward the axilla and is known as the tail of Spence (Figure 17-2).
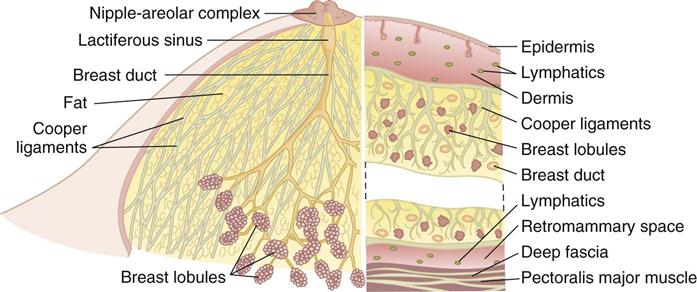
Each breast is made up of 12 to 20 glandular lobes separated by connective tissue. Each lobe drains by a single lactiferous duct that opens on the nipple. The nipple, located at about the fourth intercostal space, forms a conical projection into which the ducts open independently of each other on the surface. A pigmented circular area called the areola surrounds the nipple. Smooth muscle fibers of the areola contract to allow for nipple projection.
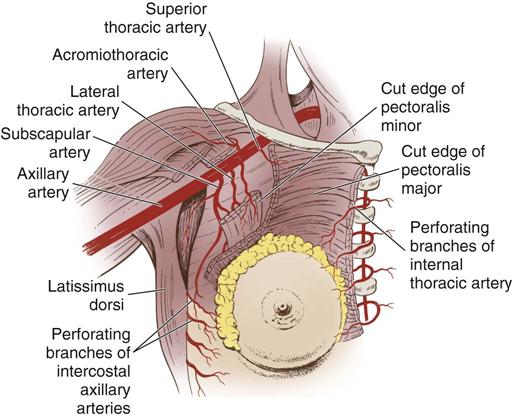
Three major arterial systems (Figure 17-3) supply the mammary glands with blood. The major supply of blood comes from the perforating branches of the internal mammary arteries, derived from the internal thoracic artery. The breast is additionally supplied by the lateral thoracic and thoracoacromial arteries as well as posterior intercostal arteries. Veins that mainly drain the breasts are the axillary along with the subclavian, intercostals, and internal thoracic veins.
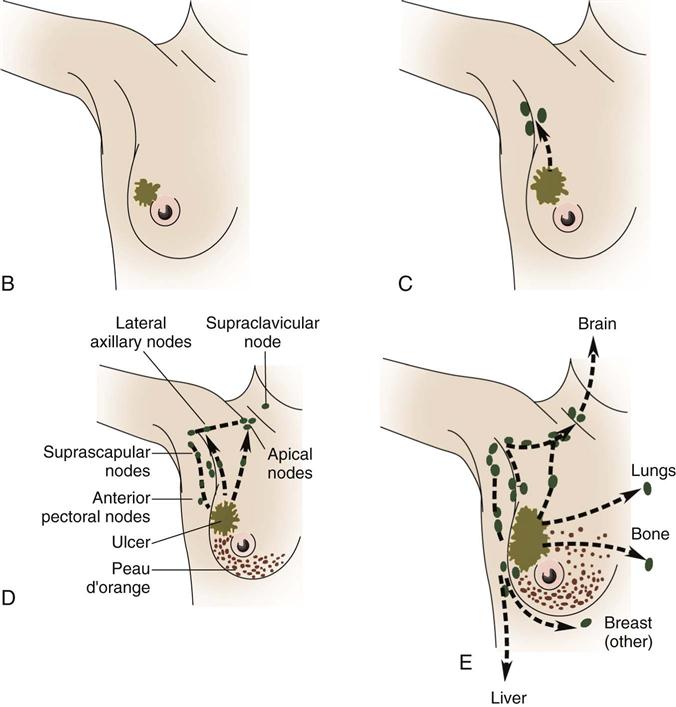
Lymph drainage generally follows the course of the vessels. Greater than 75% of lymph drainage, chiefly from the lateral quadrants, drains to the axillary lymph nodes. The remainder of lymph drains to either the parasternal nodes, the opposite breast (medial quadrants), or the inferior phrenic nodes’ lower quadrants (Figure 17-4). The internal thoracic nodes are few, but are responsible for most lymph drainage from the inner half of the breast. Thus the lymph system can also be a channel for the spread of malignant disease from the breast to associated areas of the chest wall or to the axilla.
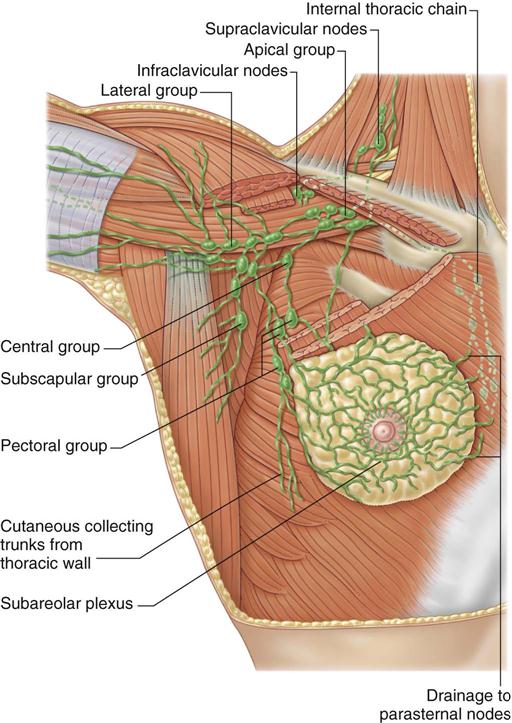
The breast’s sensory nerve supply is primarily threefold: the anterior cutaneous branches of the upper intercostal nerves, the third and fourth branches of the cervical plexus, and the lateral cutaneous branches of the intercostal nerves (Hunt et al, 2010).
The mammary glands are affected by three types of physiologic changes: (1) those related to growth and development, (2) those related to the menstrual cycle, and (3) those related to pregnancy and lactation. The mammary glands are present at birth in males and females. Hormonal stimulation, however, produces the development and function of these glands in females. Estrogen promotes growth of the ductal structures, whereas progesterone promotes lobular development. Occasionally developmental errors of the breast occur. Additional nipples or extra mammary tissue in the axilla or over the upper abdomen may be present. Absence of one or both nipples may also occur and may be associated with absence of the underlying pectoral muscle and chest wall.
Benign Lesions of the Breast
Fibrocystic change in the breast is an all-encompassing term used to describe many different breast changes. Examples of benign lesions that are generally considered when fibrocystic changes are discussed are multiple lesions of fibrous disease, intraductal papilloma, cysts, and solid masses such as fibroadenoma (Table 17-1). These changes affect almost all women at some time in their lives. Frequently pain is present, which calls attention to the problem. Pain, fluctuations in size, and multiple lesions are common features that help differentiate these generally benign lesions from cancer.
TABLE 17-1
Typical Presentation of Benign Breast Disorders
| Breast Disorder | Description | Incidence |
| Fibroadenoma | Most common benign lesion; solid mass of connective tissue that is unattached to surrounding tissue | Teenage years into 30s |
| Fibrocystic breast disease (FBD) | First stage: Characterized by premenstrual bilateral fullness and tenderness Second stage: Presence of bilateral, multicentric nodules Third stage: Presence of microscopic and macroscopic cysts | Late teens and 20s |
| Ductal ectasia | Hard, irregular mass or masses with nipple discharge, enlarged axillary nodes, redness, and edema; difficult to distinguish from cancer | Women approaching menopause |
| Intraductal papilloma | Mass in duct that results in nipple discharge: mass is usually not palpable | Women 40-55 years of age |
Modified from American Cancer Society: Noncancerous breast conditions, 2012, Atlanta, Author; Ignatavicius DD, Workman ML: Medical-surgical nursing: patient-centered collaborative care, ed 7, St Louis, 2013, Saunders; Imaginis: Non-cancerous breast conditions 2011, available at www.imaginis.com/breast-health-non-cancerous/benign-breast-conditions. Accessed January 27, 2013.
Nipple discharge is more commonly associated with benign lesions than with cancer. A postmenopausal woman who has some duct ectasia or who has borne children can manually produce nipple discharge. The most common cause of serous or serosanguineous nipple discharge is a benign intraductal papilloma, along with medications such as tricyclines and antidepressants. Discharge is usually significant only if it is spontaneous and persistent. The discharge may be clear or bloody (Townsend et al, 2012). Chronic unilateral nipple discharge, especially if bloody, should prompt an investigation for occult carcinoma.
Breast Cancer in Men
Breast cancer in men is rare; less than 1% of breast carcinomas arise in men. The average age at diagnosis is approximately 68 years. Risk factors include increased age, radiation exposure, estrogen treatment, Klinefelter syndrome, heavy alcohol intake, obesity, female relatives with a breast cancer diagnosis, and families in which breast cancer susceptibility gene 2 (BRCA2) mutation has been identified (Ohio State University Medical Center, 2013). The most common type of tumor is infiltrating ductal carcinoma. Paget disease of the nipple, inflammatory carcinoma, and intraductal cancer have also been reported in men. Symptoms include breast lumps, nipple inversion, nipple discharge, pain or pulling feelings and skin or nipple changes such as dimpling, puckering, redness, or scaling. Staging of male breast cancer is identical to staging for female breast cancer. Treatment may include surgery, radiation therapy, chemotherapy, and hormone therapy. Before hormone therapy begins, a hormone receptor test is done to determine if estrogen and progesterone receptors are present. Adjuvant therapy of radiation, chemotherapy, or hormone treatment can be given after to destroy cancer cells that cannot be detected. According to a large study of male breast cancer, male patients have more advanced disease at diagnosis, lower 5-year survival rates, and shorter median overall survival rate than women (Healthday, 2013).
Breast Cancer in Women
Breast cancer affects primarily women. Until it can be prevented, early detection is the best hope for cure. It has, in the past, been recommended that all women should practice monthly self-examination to detect palpable lesions, and they should immediately report any changes or masses to a physician. However, the efficacy of breast self-examination (BSE) in decreasing cancer mortality is being questioned. Allen and colleagues (2010) suggest patients with breast cancer risk factors such as gene mutation, family history, obstetric and gynecologic history, demographics, and environmental risk factors should be taught BSE. The U.S. Preventive Services Task Force (USPSTF) does not recommend BSE (Centers for Disease Control and Prevention [CDC], 2012). The most common form of breast cancer at present is infiltrating ductal carcinoma (Table 17-2).
TABLE 17-2
Types of Invasive Breast Cancer
| Breast Cancer Type | Percent of Breast Cancers | Specific Features |
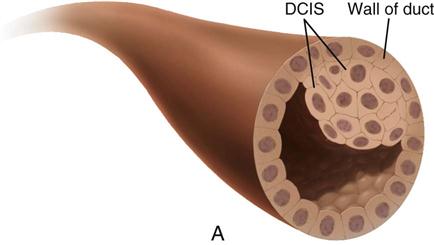
| Ductal carcinoma in situ (DCIS) | 85-90 | Can appear as microcalcifications, can vary widely in appearance, and can have features of other histologic subtypes of breast cancer. If untreated, 60%-100% of cases advance to invasive carcinoma. |
| Lobular carcinoma in situ (LCIS) | 10 | A marker for future breast cancer risk. Begins in milk (lobules) glands of breast, may become invasive, grows as single file of malignant cells around ducts and lobules and presents most often as a poorly defined mass. |
| Medullary carcinoma | 5 | Forms distinct boundary between tumor tissue and normal tissue, well circumscribed, frequent phenotype of BRCA1 hereditary breast cancer, contains lymphocytes. |
| Tubular | 2 | Infrequently metastasizes to lymph nodes, slow growing, long-term survival approaches 100%. |
| Inflammatory carcinoma | 1-5 | Rapidly growing, often with metastasis present at diagnosis, first manifestations are breast skin edema and redness and warmth with dimples or ridges. It can be confused with mastitis. More common in African American women. |
Modified from Breast Cancer.org: IDC type: tubular carcinoma of the breast 2012, available at www.breastcancer.org/symptoms/types/tubular. Accessed January 27, 2013; Townsend CM et al: Sabiston textbook of surgery: the biological basis of modern surgical practice, ed 19, Philadelphia, 2012, Saunders.
The cause of breast cancer remains unknown. Many factors, including environmental, dietary (National Cancer Institute [NCI], 2011), and familial influences have been suggested as contributors to its development (Table 17-3). The belief that breast cancer spreads by direct extension from its initial site in the breast to adjacent lymph nodes may not always be correct. Breast cancer may be a systemic condition at the time of diagnosis. Distant metastases may have already occurred without adjacent lymph node involvement at the time of palpable detection. This could explain why radical breast surgery of the past, which involved removal of the affected breast and all axillary and thoracic lymph nodes, did not greatly lower mortality. Survival from breast cancer is best when detected early, reducing axillary lymph node involvement and improving long-term survival. Tumor size can usually be correlated with involvement of lymph nodes. The larger a tumor is, the more likely it is that lymph nodes are involved.
TABLE 17-3
Risk Factors for Breast Cancer
| Factors | Comments |
| High Increased Risk (Relative Risk >4) | |
| Female gender | 99% of all breast cancers occur in women. |
| Age >50 years | Greatest risk for women occurs during ages 50-69 years. |
| Genetic factors | Inherited mutations of BRCA1 and/or BRCA2 increase risk. |
| History of previous breast cancer | Risk for developing cancer in opposite breast is five times greater than average population at risk. |
| Breast density | Dense breasts contain more glandular and connective tissue, which increases the risk for developing breast cancer. |
| Moderate Increased Risk (Relative Risk 2.1-4) | |
| Family history | One first-degree relative with breast cancer moderately increases risk. |
| Biopsy confirmed atypical hyperplasia | The overactive growth of cells increases risk. |
| Ionizing radiation | Women who received frequent low-level radiation exposure to thorax have increased risk, especially if exposure occurred during periods of rapid breast formation. |
| High postmenopausal bone density | High estrogen levels over time both strengthen bone and increase breast cancer risk. |
| Low Increased Risk (Relative Risk 1.1-2) | |
| Reproductive history (nulliparity or first child born after age 30) | Childless women have an increased risk, as do women who bear their first child near or after age 30. |
| Menstrual history (early menstruation or late menopause or both) | Risk for breast cancer rises as interval between menarche and menopause increases. Women who undergo bilateral oophorectomy before age 35 have only 40% of risk for breast cancer compared with women who undergo natural menopause. |
| Oral contraceptives | There is a slight increase in breast cancer risk in women taking oral contraceptives. |
| Hormone replacement therapy (HRT) | Use of estrogen and progesterone increases risk; routine use of hormone therapy for osteoporosis and heart disease is no longer recommended. |
| Obesity | Postmenopausal obesity (especially increased abdominal fat), increased body mass, insulin resistance, and hyperglycemia have been reported to be associated with increased risk for breast cancer. |
| Alcohol | Risk is dose dependent; consumption of 3 to 14 drinks per week is associated with a slight increase in risk; risk increases with increased consumption. |
| High socioeconomic status | Breast cancer incidence is greater in women of higher education and socioeconomic background. This relationship is possibly related to lifestyle differences, such as age at first birth. |
| Jewish heritage | Women of Jewish Ashkenazi heritage have higher incidences of BRCA1 and BRCA2 genetic mutations. |
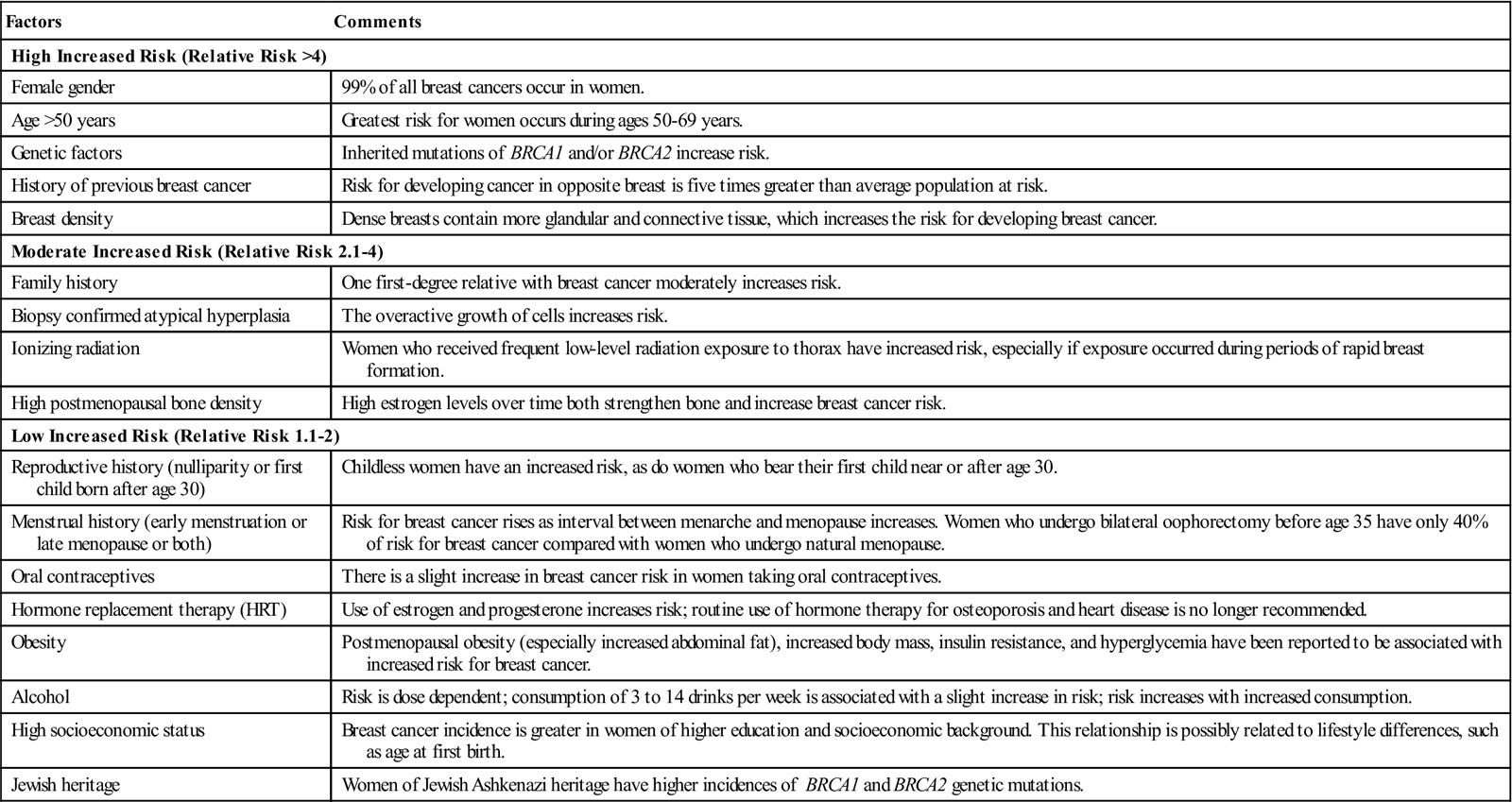
Modified from American Cancer Society: Breast cancer facts and figures 2010-2011, Atlanta, 2012, Author; Ignatavicius DD, Workman ML: Medical-surgical nursing: patient-centered collaborative care, ed 7, St Louis, 2013, Saunders.
Less radical surgery is the treatment of choice today. Most small noninvasive and invasive breast cancers are treated by breast conservation therapy (BCT). Surgical excision of the tumor, the use of radiation therapy alone, or a combination of surgery, chemotherapy, and radiation therapy are treatment recommendations. The use of adjuvant chemotherapy is particularly recommended for premenopausal women with axillary node metastasis. Studies show that similar therapy can benefit node-negative breast cancer patients. New studies and new therapeutic options are continually being developed and tested.
Clinical use of accelerated partial breast irradiation (APBI) is a current treatment. In APBI the radiation is focused on the area of greatest risk for tumor recurrence, the lumpectomy site, instead of whole breast irradiation (WBI). Such focused radiation treatment therapies reduce the traditional 6-week radiation treatment plan to 1 week to 3 weeks (ACS, 2012). Although external beam radiation therapy of the whole breast after lumpectomy has been the standard of care for treating early stage breast cancer, APBI is being used increasingly as an alternative treatment option for carefully selected patients.
Brachytherapy, also known as internal radiation, is another delivery method of radiation. Radioactive seeds are placed precisely into the adjoining cancerous breast tissue. There are two kinds of brachytherapy: interstitial and intracavitary. In interstitial brachytherapy, many small, hollow catheters are inserted into the breast around the lumpectomy area and are left in place for several days. Radioactive pellets are inserted into the catheters for short periods each day and then removed. It is not as common today as intracavitary brachytherapy, which is a form of APBI that involves placing a source of radiation into the lumpectomy site for a short period and removing it. It is usually administered twice a day for 5 days on an outpatient basis (ACS, 2012). Clinicians are likely to prefer the newer intracavitary brachytherapy technique for APBI because this method is less invasive and less technically demanding than interstitial techniques.
Minimally invasive cryoablation technology to freeze and destroy solid breast tumors is another option for treatment of a breast tumor. Another breast surgery alternative is focused ultrasound (FUS) with magnetic resonance guidance (MRgFUS), which allows for imaging and tissue temperature monitoring while controlling the FUS beam direction during ablation of breast lesions (Tempany et al, 2011).
Screening Technologies
Imaging methodologies, such as mammography and ultrasonography (US), have helped detect breast masses too small for clinical detection. A recent study supports the need to identify and track breast cancer screening disparities in the United States (Research Highlight). The American Cancer Society (ACS) recommends clinical breast exams by a physician every 3 years for women ages 20 to 39 years, with annual clinical breast exams and mammograms for women 40 and older (Table 17-4). Women who are at high risk should have magnetic resonance imaging (MRI) and a mammogram every year. High-risk women include those who have a known breast cancer susceptibility gene 1 (BRCA1) or breast cancer susceptibility gene 2 (BRCA2) mutation, those who have a first-degree relative with a BRCA1 or BRCA2 gene mutation, and those who have a history of breast cancer, ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), atypical ductal hyperplasia, or atypical lobular hyperplasia (ACS, 2012). The USPSTF advises women ages 50 years and older to have a screening mammogram every 2 years (CDC, 2012). Many professional organizations such as the American Medical Association, American College of Surgeons, American College of Obstetricians and Gynecologists, and the American College of Radiology advocate mammography to begin at age 40.
TABLE 17-4
American Cancer Society Breast Cancer Screening Guidelines for Asymptomatic Women*
| Age | Screening Activity |
| 20-39 yr | Breast self-examination (BSE) optional |
| Clinical breast examination (CBE) every 3 yr | |
| 40 yr and older | BSE optional |
| CBE annually prior to mammogram | |
| Annual mammogram |
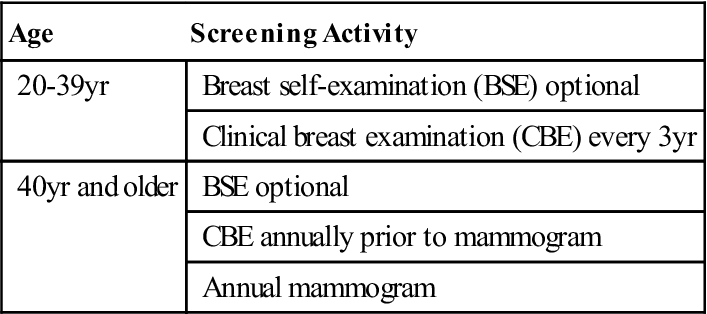
*Asymptomatic women identified to be at higher risk need to have individualized screening plans that may differ from these guidelines.
Modified from American Cancer Society: Breast cancer facts and figures 2011-2012, Atlanta, 2012, Author, available at www.cancer.org/research/cancerfactsfigures/breastcancerfactsfigures/breast-cancer-facts-and-figures-2011-2012. Accessed January 27, 2013.
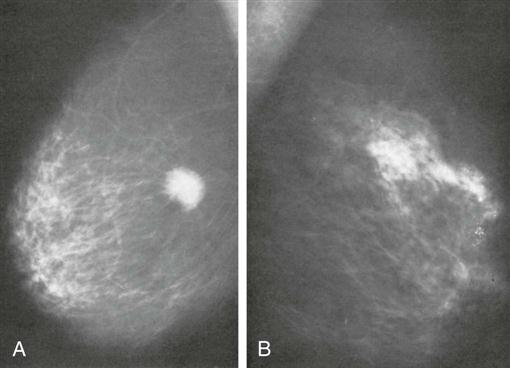
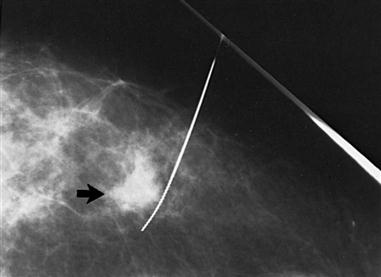
The most common screening mechanism for asymptomatic women is mammography (Figure 17-5). In mammography the entire breast is visualized as x-ray beams are directed in several planes through it. Mammograms detect abnormal-appearing densities, irregular or spiculated margins, microcalcifications, and clusters of calcium deposits that are clinically nonpalpable. Screening mammography is the most helpful in early detection because it can discover cancer several years before physical symptoms such as a lump develop. Often previous mammograms are used for comparison. The accuracy of mammography depends on careful x-ray technique and breast size, structure, and density. Radiation dosage varies with individuals and techniques. As a result of improved radiologic techniques, radiation exposure in a mammogram is very low. The benefits of this screening mechanism far outweigh the minute risks of radiation exposure. Advances in computer-assisted detection allow the computer to analyze the mammogram, placing asterisks and triangles on small potential problem areas, which a radiologist then reviews. In some instances, such as when a lesion previously detected in a mammogram is too small to palpate, mammograms are repeated immediately before surgery. The lesion is localized by the insertion of a needle or a wire within a needle (often referred to as “needle” or “wire” localization) (Figure 17-6). If screening mammography reveals a suspicious area, the patient is asked to return for diagnostic mammography.
Digital mammography, also known as full-field digital mammogram (FDDM), is similar to a standard mammogram in that an x-ray is used to take images of the breast. The mammograms are recorded and viewed on a computer where the image size, brightness, or contrast in certain areas of the film can be adjusted and seen more plainly. Digital images can be sent electronically to other sites for viewing and consulting with breast specialists. Radiologists also can use software to interpret digital mammograms. Digital screening mammograms are more accurate than film screening mammograms, with a 70% detection rate compared with 55% for film screens. Women most likely to benefit from digital screening are those younger than 50 with dense breast tissue and who are premenopausal or perimenopausal (ACS, 2012). When a palpable mass or other abnormality is identified on screening mammography, additional diagnostic mammographic views are obtained, and the radiologist assigns a Breast Imaging Reporting and Data System (BI-RADS) score (Box 17-1). US may then be ordered.
US differentiates between solid and cystic lesions. A cyst is a fluid-filled sac and is considered a benign finding. A solid mass may suggest malignancy and requires further evaluation. As a screening methodology alone, its sensitivity and specificity are less definitive than mammography. US can be useful with dense or dysplastic breasts and in pregnant or lactating women. Women 30 to 39 years of age benefit most from US, which has a sensitivity of 95.7% compared with 60.9% for mammography and discovers more cancerous lesions than mammography (Lehman et al, 2012).
MRI is used as an adjunct to mammography in the detection of breast lesions. MRI uses a strong magnet to produce a complete image of the breast. There is no radiation. Breast MRI can image dense breast tissue, which shows poorly with conventional mammography. The ACS recommends that MRI be used for women at high risk. Such women include those who have known breast cancer–associated BRCA1 or BRCA2 gene mutations, have a first-degree relative with a BRCA1 or BRCA2 gene mutation, have a lifetime risk of breast cancer of 20% to 25% or greater according to risk assessment tools, and had radiation therapy to the chest between 10 and 30 years of age. MRI also better determines definite size of the cancer and is useful in women with breast augmentation.
Molecular breast imaging (MBI) refers to a number of modalities that use a radiopharmaceutical agent in scanning, including breast-specific gamma imaging (BSGI) and positron emission mammography (PEM). It involves injection of a short-lived radiotracer, which is absorbed by breast tissue and preferentially so by breast tumors. MBI is especially useful in detecting breast cancer in women with dense breasts, those who present with a palpable abnormality not found on mammogram or US, and those who cannot tolerate MRI or who have metallic implants (Kaplan, 2011).
Deoxyribonucleic acid (DNA)–based genetic testing for BRCA1 and BRCA2 is recommended for women with family histories that suggest risk for these gene mutations. These mutations lead to the development of hereditary breast and/or ovarian cancer in affected women. Individuals with a harmful BRCA mutation have a lifelong chance of up to 80% risk of developing breast cancer and are more likely to be diagnosed before menopause (Breast Cancer.org., 2012). To test for BRCA1 and BRCA2, a blood DNA sample is needed for full-length gene sequencing. A genomic profiling tool for breast cancer, approved in the United States, is specifically for estrogen-receptor–positive, node-negative breast cancer. One of the test results is a recurrence score, which correlates the chance of a specific tumor returning in the next 10 years. Genetic counseling is suggested before and after the workup (Townsend et al, 2012).
Diagnostic Techniques
Once a mass is identified, the physician has multiple techniques to establish a diagnosis. During a fine-needle aspiration biopsy (FNAB) the physician or radiologist anesthetizes a small area of the breast with lidocaine. FNAB is mainly used for differentiation of solid from cystic masses but may also be performed when a new, dominant, unexplained mass is found in the breast. A 22- or 25-gauge needle attached to a 20-mL syringe is inserted into the mass, and a small amount of the contents is aspirated, then prepared on a slide for cytologic examination. Cyst fluid is usually cloudy and dark green or amber. If the mass is solid, the needle is repetitively inserted into the mass while steady negative pressure is applied to the syringe; suction is released and the needle is withdrawn. The slides are then fixed and submitted to pathology. Cytologic evaluation does not reveal if the cancer is invasive or noninvasive, so most physicians advise a core needle biopsy for histologic diagnosis before a surgical procedure (Townsend et al, 2012).
A core needle biopsy is similar to a FNAB but a larger needle is used because actual breast tissue is removed rather than a sampling of cells. Core needle biopsy is preferred over excisional biopsy because it is minimally invasive and less expensive (Townsend et al, 2012). The types of core biopsies are US-guided core biopsy and stereotactic core biopsy. US-guided core biopsy does not require surgery. A large biopsy needle is placed into the breast tissue and the US aids in confirming the proper needle placement for biopsy. US can also distinguish many benign lesions such as fluid-filled cysts. Tissue samples are then obtained through the needle. In stereotactic core biopsy, a large biopsy needle is inserted into the breast tissue; computerized mammographic pictures facilitate needle placement using digital imaging so the precise location of breast tissue is biopsied. Tissue samples are then withdrawn through the needle (Cleveland Clinic, 2009).
Surgical Treatment Options
Surgical treatment ranges from minimally invasive breast biopsy, BCT, wide excision of the tumor mass, or modified radical mastectomy involving the breast and axillary lymph nodes. In some cases, salpingo-oophorectomy is considered (Domchek et al, 2010). BCT surgeries are alternately called segmental mastectomy, lumpectomy, partial mastectomy, wide local excision, and tylectomy (Hunt et al, 2010). The goal of surgery is removal of the cancerous mass with a margin of normal tissue and a good cosmetic result. When a specimen of breast tissue is sent to the laboratory, it is inked to mark the relationship between the tumor and the surgical margins of the excision. The pathologist evaluates these margins on all sides of the tumor for malignant cells. If a margin is positive it indicates that malignant cells may remain in the breast. Additional surgery is usually required until the margins contain only normal tissue (Stephan, 2009).
The choice of procedure depends on the size and site of the mass, the characteristics of the cells, the stage of the disease, and the patient’s choice. A breast cancer diagnosis is usually staged to measure the extent of the disease and to classify patients for possible treatment modalities (Figure 17-7 and Table 17-5). The TNM (T = tumor; N = node; M = metastasis) classification has been adopted as a mechanism to clinically stage this disease. Staging results are used in designing a specific treatment plan. Radiation therapy, chemotherapy, or hormonal therapy may be used with surgery or as alternative treatment methods. An intravenous (IV) access port may be surgically placed for later infusion of chemotherapeutic medications, fluids, or nutrition as well as to withdraw blood and laboratory specimens (Ambulatory Surgery Considerations).
TABLE 17-5
| Stage 0 | Carcinoma in situ LCIS—abnormal cells in lining of a lobule DCIS—intraductal carcinoma; abnormal cells that are in lining of a duct |
| Stage I | Tumor <2 cm with no axillary lymph node involvement |
| Stage IIa | Cancer cells found in axillary lymph nodes, or tumor is <2 cm with metastasis to axillary lymph nodes, or tumor >2 cm but not >5 cm with no metastasis to axillary lymph nodes |
| Stage IIb | Tumor >2 cm but not >5 cm with positive axillary lymph nodes or tumor >5 cm with negative axillary lymph node involvement |
| Stage IIIa | Tumor ≤5 cm with metastasis to axillary lymph nodes that are attached to each other or to other structures or has spread to lymph nodes behind the breast bone; or tumor ≥5 cm with spread to axillary lymph nodes that are alone or attached to each other or to other structures or has spread to lymph nodes behind the breast bone |
| Stage IIIb | Tumor of any size that has grown into the chest wall or skin of the breast causing swelling of the breast or nodules in breast skin and may have spread to axillary lymph nodes that are attached to each other and to other structures and may have spread to lymph nodes behind the breast bone |
| Stage IIIc | Tumor of any size that has spread either to the lymph nodes behind the breast bone and axillary lymph nodes or to the lymph nodes above or below the collarbone |
| Stage IV | Distant organs |
DCIS, Ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Modified from Breastcancer.org: Stages of breast cancer, available at: www.breastcancer.org/symptoms/diagnosis/staging.jsp. Accessed January 27, 2013; National Cancer Institute at the National Institutes of Health: Breast cancer treatment (PDQ), available at www.cancer.gov/cancertopics/pdq/treatment/breast/Patient/page2. Accessed January 27, 2013.
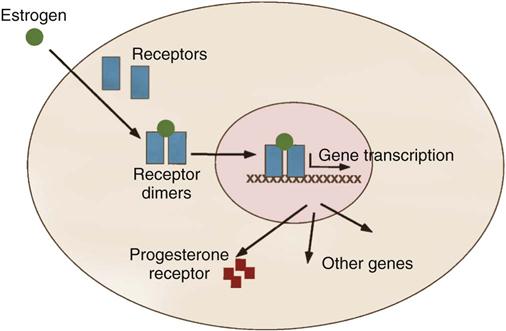
Excised tumors or core needle biopsies are evaluated for their estrogen-binding and progesterone-binding abilities. Techniques have been developed to assess the ability of breast cancer to bind with estrogen and progesterone. This positive binding capability identifies the patient with a hormone-dependent tumor (Figure 17-8). It is estimated that about two thirds of all breast cancers are positive for estrogen binding, a majority of which are also positive for progesterone binding. The presence of these receptor sites is favorable to hormone manipulation, with the goal of preventing breast cancer cells from receiving estrogen stimulation. In these patients, who also have negative axillary nodes, it is decidedly preferable to obtain a genomic profile of the primary cancer to find out the risk for recurrence (Townsend et al, 2012). Use of selective estrogen receptor modulators (SERMs) and estrogen receptor down-regulators (ERDs), in addition to surgery and chemotherapy, increase disease-free survival in premenopausal women with positive binding for estrogen. Postmenopausal women benefit from aromatase inhibitors (AIs). They appear to work better than estrogen receptor modulators on certain breast cancers, with fewer side effects.
Some of the most promising data reported for advanced breast cancer involves human epidermal growth factor receptor 2 (HER-2), a cellular proto-oncogene coding for a transmembrane receptor. Agents such as trastuzumab (Herceptin), to target HER-2 were first approved for treatment of metastatic HER-2–positive breast cancer in 1998. In 2012, pertuzumab was approved for use in combination with trastuzumab and with the chemotherapy agent docetaxel (Baselga et al, 2012). It is indicated for use in patients with metastatic HER-2–positive breast cancer who have not received previous treatment with either chemotherapy or HER-2–targeted therapy. Research and understanding of the immune system, development of methods to evaluate aspects of the immune response, and ongoing development of monoclonal antibodies continue to transform the field of immunotherapy and breast cancer treatment (Modi, 2011).
Clinical trials are exploring the clinical effectiveness of poly ADP-ribose polymerase (PARP) enzyme inhibitors (Surgical Pharmacology) in triple-negative breast cancer (TNBC) patients. These tumors are estrogen and progesterone hormone receptor negative and HER-2/neu negative. TNBC is found in younger women, women of African American or Hispanic descent, and individuals with BRCA1 mutations. It also is associated with a worse prognosis than other breast cancer subtypes (Winer, 2011).
Surgical Pharmacology
Drugs Used in Breast Cancer
| Medication Category | Dosage/Route | Purpose/Action | Adverse Reactions | Nursing Implications |
| Anastrozole Functional class: Antineoplastic Chemical class: Aromatase inhibitor | 1 mg daily by mouth | Advanced breast cancer not responsive to other treatment in estrogen receptor–positive patients (usually postmenopausal), patients with advanced disease on tamoxifen, adjunct treatment in early breast cancer. Highly selective nonsteroidal aromatase inhibitor, lowers serum estradiol concentrations | Hot flushes, headaches, lightheadedness, nausea, vomiting, rash, leukopenia, chest pain, hypertension, edema, angina, myocardial infarction, cerebral infarct | Stevens-Johnson syndrome; take calcium and vitamin D for bone loss/fracture; increase in size of tumor and increased bone pain may occur and will subside rapidly. |
| Exemestane Functional class: Antineoplastic Chemical class: Aromatase inhibitor | 25 mg by mouth after meals; may need 50 mg/day if taken with a potent CYP3A4 inducer | Advanced breast cancer not responsive to other therapies. Lowers serum estradiol concentrations | Headache, depression, insomnia, anxiety, fatigue, hot flushes, nausea, lymphopenia, dyspnea | Assess blood pressure for hypertension and bone mineral density; use reliable contraception; do not breastfeed. |
| Fulvestrant Functional class: Antineoplastic Chemical class: Estrogen receptor antagonist | 500 mg on days 1, 15, 29, and monthly thereafter as an IM injection | Advanced breast cancer in estrogen receptor–positive patients | Headache, nausea, vomiting, rash | Encourage nutritious diet with iron and vitamins; store in refrigerator; report vaginal bleeding immediately; increase in size of tumor; use contraception. |
| Letrozole Functional class: Antineoplastic, nonsteroidal aromatase inhibitor | 2.5 mg daily by mouth | Early advanced or metastatic breast cancer in postmenopausal women | Headache, lethargy, nausea, vomiting, anorexia, rash, pruritus, myocardial infarction (MI), cerebrovascular accident (CVA), endometrial carcinoma, vaginal bleeding, second malignancies | Regular assessment of hepatic function studies; may administer with bisphosphonates to increase bone density; nutritious diet with iron and vitamins; report vaginal bleeding. |
| Poly ADP-ribose polymerase (PARPs) inhibitors Functional class: Antineoplastic | Varies; 200 mg bid | Treat triple-negative breast cancer patients. Causes the BRCA genes to function improperly because of the mutation | Fatigue, somnolence, mood alteration | Report anything that may be considered a side effect; very little reported. |
| Raloxifene Functional class: Bone reabsorption inhibitor Chemical class: Hormone modifier, selective estrogen receptor modulator | 60 mg daily by mouth | Breast cancer prevention, treatment of osteoporosis in postmenopausal women, breast cancer prophylaxis. Reduces resorption of bone and decreases bone turnover; mediated through estrogen receptor binding | Nausea, hot flushes, leg cramps, CVA, pulmonary embolism (PE), insomnia, peripheral edema, fever, weight gain | Regular hepatic studies done during therapy; bone density baseline obtained; administer vitamin D, calcium supplement; weigh weekly and report 5-lb weight gain; avoid staying in one position for long periods. |
| Tamoxifen Functional class: Antineoplastic Chemical class: Antiestrogen hormone | Breast cancer: 20 to 40 mg daily by mouth for 5 years High-risk breast cancer and DCIS: 20 mg daily by mouth for 5 years | Prevention of breast cancer following surgery or radiation; advanced breast cancer not responsive to other therapies in estrogen-positive patients. Inhibits cell division by binding to cytoplasmic estrogen receptors | Hot flushes, headache, lightheadedness, nausea, vomiting, rash, stroke, uterine malignancy, thrombocytopenia, PE, uterine malignancy, leukopenia | Do not break, crush, or chew tablets; obtain complete blood count (CBC) with differential and platelets regularly; encourage nutritious diet; increase fluids; routine eye exams. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree