Breast Reconstruction Following Mastectomy
James N. Long
John B. McCraw
Jorge I. de la Torre
Luis O. Vásconez
As reconstructive surgeons, we are tasked with recreating that which has been lost. Indeed our history shows an unending search for the most ideal means by which to accomplish “restoration of form.” In the context of modern breast oncology, we are called on to recreate a breast mound that is aesthetic in its shape and contour while also achieving symmetry with the unoperated side. These goals must be accomplished without handicapping any future treatment for breast cancer while keeping the operation manageable, reproducible, and most importantly safe for the patient. Surgical changes in the modes of treatment for breast cancer have been dramatic since Halsted described his radical mastectomy. We now strive to diagnose disease in its earliest stages, which has made possible modern breast-conserving therapies. Modern breast reconstructive techniques are in a continuous state of evolution, attempting at every turn to improve cosmesis and function while reducing morbidity. Presently our task is made easier due to the smaller defects produced by newer ablative oncologic techniques. Until the late 1970s to early 1980s, reconstruction of breast was mostly done as a delayed procedure, months or even years after mastectomy. At that time, a number of concerns in performing immediate breast reconstruction existed. First and foremost among these concerns was the question of oncologic safety in immediate reconstruction, especially with the idea of reconstructed tissues hiding local cancer recurrence. In addition, there was concern over possible seeding of breast tumors throughout the chest or donor site. Some surgeons even posited that immediate breast reconstruction could interfere with adjuvant chemotherapy, whereas others feared these lengthy one-stage procedures would increase complication rates.
The advantages of immediate reconstruction are now quite clear and one very important improvement is found in not having to surgically re-expose the mastectomy
site to achieve reconstruction. Furthermore, the patient sustains an emotional benefit from the one-stage procedure, which also serves to diminish the total length of treatment. Many studies have now been completed that have sought to definitively address those concerns surrounding immediate breast reconstruction. From an oncologic safety perspective, most local recurrences are now known to occur either at the subcutaneous tissue margins or along the scar within about 3 years after mastectomy. This has been found to be true even in the patients who have received immediate breast reconstruction. Consequently, recurrences are usually detected with palpation or inspection in both reconstructed and non-reconstructed groups. To address concerns over putative seeding of the donor site, reconstructive surgeons, in cooperation with their oncologic surgery colleagues, have settled on a system, which uses separate surgical teams; this practice includes the use of different instruments, nurses, and surgical staff. To allay concerns over the prospect of immediate reconstruction causing delay in the initiation of adjuvant chemotherapy, a study was performed at the University of Alabama at Birmingham (UAB) comparing a group of 125 patients who underwent immediate reconstruction with a similar group of patients who did not undergo reconstruction. This study demonstrated that the great majority of patients who underwent immediate reconstruction were able to initiate adjuvant chemotherapy within 6 weeks of mastectomy and reconstruction. Counterintuitive to the initial presumption, a significant percentage of patients undergoing mastectomy without reconstruction experienced delayed initiation of adjuvant chemotherapy beyond 6 weeks as a result of the persistence of seromas along the axilla or from mastectomy flap necrosis. The overall complication rates for these two groups of patients were identical (18%).
site to achieve reconstruction. Furthermore, the patient sustains an emotional benefit from the one-stage procedure, which also serves to diminish the total length of treatment. Many studies have now been completed that have sought to definitively address those concerns surrounding immediate breast reconstruction. From an oncologic safety perspective, most local recurrences are now known to occur either at the subcutaneous tissue margins or along the scar within about 3 years after mastectomy. This has been found to be true even in the patients who have received immediate breast reconstruction. Consequently, recurrences are usually detected with palpation or inspection in both reconstructed and non-reconstructed groups. To address concerns over putative seeding of the donor site, reconstructive surgeons, in cooperation with their oncologic surgery colleagues, have settled on a system, which uses separate surgical teams; this practice includes the use of different instruments, nurses, and surgical staff. To allay concerns over the prospect of immediate reconstruction causing delay in the initiation of adjuvant chemotherapy, a study was performed at the University of Alabama at Birmingham (UAB) comparing a group of 125 patients who underwent immediate reconstruction with a similar group of patients who did not undergo reconstruction. This study demonstrated that the great majority of patients who underwent immediate reconstruction were able to initiate adjuvant chemotherapy within 6 weeks of mastectomy and reconstruction. Counterintuitive to the initial presumption, a significant percentage of patients undergoing mastectomy without reconstruction experienced delayed initiation of adjuvant chemotherapy beyond 6 weeks as a result of the persistence of seromas along the axilla or from mastectomy flap necrosis. The overall complication rates for these two groups of patients were identical (18%).
Breast reconstruction was conceived to address defects of anatomy, and for this reason it is not accurate to consider its implementation as cosmetic surgery but rather as an endeavor to achieve “restoration of form.” Cosmetic surgery implies intent to enhance a patient’s normal appearance rather that to restore it. Of course, it is expected that any reconstructive breast procedure would serve to improve the appearance when referenced against an unreconstructed mastectomy site or radiation injury, but not when weighed against normal anatomy.
When we look to the past, we know that patients with breast cancer were not routinely considered for reconstruction at the time of mastectomy. Although sound rationale for this position existed then, such as larger tumors and suboptimal methods of reconstruction, these concerns are not usually germane in the current era. We now practice in systems that offer both earlier diagnosis and improved methods of reconstruction that are consistently reproducible and yield satisfactory results. Most mastectomies today are performed for totally curable lesions such as in situ, multi-focal, and minimally invasive breast disease. Out of these advances, we now favor routine breast reconstruction at the time of mastectomy for the patients with stages 0, I, and IIa. Presently, this subset circumscribes the vast majority of patients undergoing mastectomy. Indeed, there presently exists no scientific basis for denying these patients immediate reconstruction.
When immediate reconstruction is not offered in this current surgical era, it most typically occurs because of a lack of surgical resources at the facility or out of deference to now archaic conventions. Women remain no different from men in their desire to maintain their form; and now, due to the effort of countless patients and surgeons, it is increasingly rare that a woman who chooses to undergo breast reconstruction is denied such opportunity. In the past, many had argued that breast reconstruction was cosmetic and thus non-essential, since reconstructing the breast does not add to the patient’s function. These attitudes have been replaced by an understanding that restoring the patients’ form does have a significant positive impact on their self-perception, allowing greatly facilitated reintegration into their premorbid life. Since the advent of managed care, breast reconstruction in women has moved from having no financial justification to, in most cases, requiring none. When tumor extirpation causes a physical deformity, treating surgeons should routinely consider reconstruction, understanding that now it is of benefit to their patients and in most cases approved by third-party payers. While breast reconstruction serves as a critical component of the overall process of cancer rehabilitation, it is also vital to restore the patient physically, emotionally, and spiritually. In addition to the psychological benefits realized from reconstruction, the methods used to achieve these aims have also shown themselves advantageous in the treatment and prevention of radiation injuries of the breast and chest wall. It is clearly recognized that radiation therapy causes a vasculitis that can progressively change skin, muscle, and bone over time. Higher radiation dosages, particularly when concentrated in a localized area, may result in fibrosis of the pectoralis muscle and ischemic necrosis of the costal cartilages and sternum resulting in late disruption of the overlying skin. Though radiation injuries are less common now than in the past, they still occur in spite of continuous refinements in technique. This is important to consider because in younger women the progressive vasculitis of radiation therapy may cause problems 20 or 30 years after treatment. No matter how carefully performed, radiation therapy will produce a quantity of ulcers, lumpectomy-radiation failures, and other problem wounds that can be best corrected by introducing well-vascularized tissue to the damaged area. When resection of the chest wall is indicated, myocutaneous flaps are known to be indispensable in achieving a safe, durable closure of the wound.
The Ideal Total Mastectomy
From the perspective of the reconstructive surgeon, the ideal total mastectomy should preserve the pectoralis major muscle and all of the skin that is not essential to tumor resection. When this approach is used, the functional preservation of the shoulder and soft tissue coverage of the ribs is achieved. Extensive skin excision results in a tight closure and is not shown to enhance patient’s survival. The removal of this redundant native tissue does however make reconstruction significantly more difficult. Tissue expanders cannot overcome a large skin deficit and the need for more complex autologous flap reconstruction is virtually assured when a mastectomy with radical skin resection is performed. The difference between laxity and tightness is usually a difference of about 2 cm of additional skin with each mastectomy flap. The addition of 4 to 6 cm of skin to the closure is remarkably helpful to any intended reconstruction and is often a determining factor in whether a myocutaneous flap will be required to correct the deformity.
The placement of incisions is the other key factor that determines if mastectomy scars will be favorable. Here, the leading consideration should be placing incisions along the lines of skin tension. To identify the direction of these lines, pinch the skin, which accentuates the wrinkles native to the resting lines of skin tension. When incisions adhere to these lines precisely, healing occurs with the best chance of avoiding prolonged redness as well as widened or hypertrophic scarring. We favor the complete skin-sparing periareolar incision, with superior-lateral extension when needed and where practicable. When prior biopsy sites
preclude the use of skin-sparing option, an elliptical incision is chosen to include the nipple, areola, and prior biopsy site. Either will provide adequate access for any mastectomy. Once complete with resection, we retain all skin redundancy in the closure to assist in any delayed reconstruction. The worst choice for a closure in a patient who will eventually have a breast reconstruction is the transverse Patey incision. It is inelastic and unexpandable and results in the least attractive scars (Fig. 1A,B,C).
preclude the use of skin-sparing option, an elliptical incision is chosen to include the nipple, areola, and prior biopsy site. Either will provide adequate access for any mastectomy. Once complete with resection, we retain all skin redundancy in the closure to assist in any delayed reconstruction. The worst choice for a closure in a patient who will eventually have a breast reconstruction is the transverse Patey incision. It is inelastic and unexpandable and results in the least attractive scars (Fig. 1A,B,C).
Partial Mastectomy
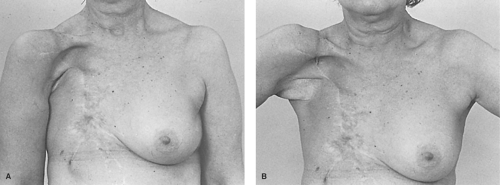
Occasionally still unrecognized is that any partial breast deformity is preferable to a complete one. Replacement of up to 50% of the breast volume without the need for skin is a relatively simple reconstructive challenge. This can be accomplished with an autologous latissimus flap in a single stage. The usual skin defect of the non-skin-sparing mastectomy, which approximates 7 cm by 15 cm, can also be readily corrected with the latissimus flap. The favorable nature of the partial defect is a sound reason to choose lumpectomy and radiation when indicted by tumor size and location. When the planned lumpectomy is anticipated to cause breast deformity, even when planning to remove the nipple, breast-conserving therapy is still tenable with good aesthetic outcomes using the latissimus or its perforator variants. Although a partial central mastectomy will deform the breast, the deformity of a complete mastectomy will always be worse than this partial defect. The loss of the central breast and nipple are relatively simple to reconstruct and can be done before or after radiation.
The skin-sparing mastectomy limits the skin excision adequate for tumor removal, which includes the nipple, the biopsy site, and any breast skin that is within 1 to 2 cm of the tumor. In most patients with stage I and IIa tumors, this approach is reasonable since tumors are more often deep than they are close to the skin. Even when the tumor lies beneath the nipple, a wide skin ellipse is usually unnecessary. In such cases, the periphery of the ellipse or the margin of tumor excision can be upward 10 to 15 cm, far in excess of that which is required to adhere to any treatment protocol. A circular excision of the nipple/areola complex will typically provide satisfactory margins and exposure with far less impediment to a reasoned reconstructive plan. Skin conservation facilitates reconstruction by all methods and greatly improves the aesthetic result that can be achieved by autologous tissue. Even in cases where reconstruction will never be performed, there is no reason to excise the skin widely, except to achieve oncologic clearance. The reconstructive surgeon should not be a solitary voice in encouraging skin conservation. It is in the patient’s best interest to avoid radical skin excision when glandular resection and limited skin excision provides the same margin of safety.
Modified Radical Mastectomy with Extensive Skin Excision
Until the early 1990s when advocates for skin-sparing mastectomy began to be heard, the mastectomy skin excised with the modified radical technique differed
little from the standard radical mastectomy. Extensive skin excision became a cornerstone of mastectomy when Handley promoted the permeation theory of centrifugal tumor spread in 1915. Some practicing surgeons today still model their modified radical mastectomy after the description of Patey, employing radical skin excisions with the primary closure—a perceived necessity also promoted by Auchincloss and Madden. Their preferred methodology closely followed the original description given by Moore in 1867. Patey espoused the belief that removal of the pectoralis major muscle contributed little to the cure of breast cancer since the tumor seldom invaded the muscle, except in the case of close margins. Instead, skin permeation with tumor became his primary concern in tumor extirpation and so he attempted primary closure in only about half of his cases. Compared with the radical mastectomy, the Patey mastectomy is less radical with regard to pectoralis major muscle but just as radical in elimination of skin.
little from the standard radical mastectomy. Extensive skin excision became a cornerstone of mastectomy when Handley promoted the permeation theory of centrifugal tumor spread in 1915. Some practicing surgeons today still model their modified radical mastectomy after the description of Patey, employing radical skin excisions with the primary closure—a perceived necessity also promoted by Auchincloss and Madden. Their preferred methodology closely followed the original description given by Moore in 1867. Patey espoused the belief that removal of the pectoralis major muscle contributed little to the cure of breast cancer since the tumor seldom invaded the muscle, except in the case of close margins. Instead, skin permeation with tumor became his primary concern in tumor extirpation and so he attempted primary closure in only about half of his cases. Compared with the radical mastectomy, the Patey mastectomy is less radical with regard to pectoralis major muscle but just as radical in elimination of skin.
In the normal breast, there exists no tightness of either the skin or pectoralis major muscle and 180 degrees of shoulder abduction is the normal full range of motion. By comparison, the skin closure of the standard modified radical mastectomy typically causes skin tension at 90 degrees of shoulder abduction followed by limitation in shoulder motion beginning at 130 degrees of abduction (Fig. 2). In practice, the elliptical skin excision usually measures 10 cm by 17 cm, which is easily sufficient to result in skin contracture across the chest. A horizontal skin ellipse has the most deleterious effect on shoulder motion when compared to other incisions because of tethering of normally expansile skin at a site between the inframammary fold and the axilla. The usual transverse skin closure is also the most unfavorable scar for breast reconstruction since it crosses the skin tension lines perpendicularly, usually resulting in scar hypertrophy and mastectomy flap stiffness. The low, oblique scar is much more favorable as the scar tethers at a site normally fixed to the chest wall, the inframammary fold. Though still suboptimal, the vertical closure is less restrictive than the transverse.
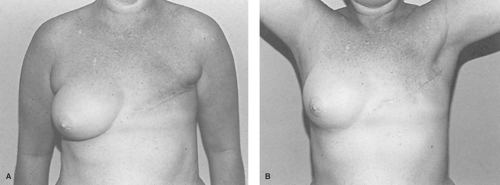
Reconstruction of a patient who has had a modified radical mastectomy with extensive skin excision is equal in difficulty to reconstructing the one with a radical mastectomy deformity. Reconstruction in these patients cannot be accomplished by means of an implant or a tissue expander alone since all surrounding skin has been “recruited” to achieve closure of the mastectomy defect. Autologous skin flaps are then required to replace lost breast skin as tissue expanders cannot overcome the tightness of the initial skin closure.
Radical Mastectomy
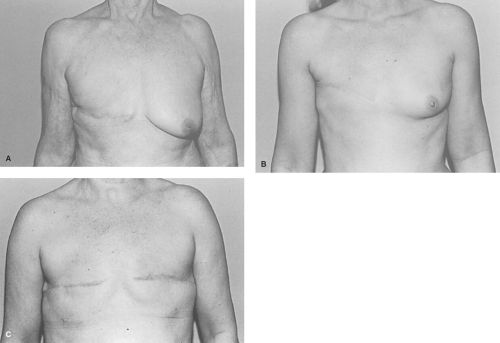
In the radical mastectomy skin resection is always extensive and resultantly produces chest wall tightness, visible ribs, and compromise of shoulder mobility. Radical mastectomies are rarely seen today, thanks to earlier diagnosis and an improved understanding of the natural history of breast cancer. Nevertheless, patients still present with these massive deformities created years ago when it was considered the standard of care (Fig. 3). Because of the inadequate soft tissue cover, in most cases it is impossible for patients to wear an external prosthesis. Reconstruction of this deformity is clearly not possible by means of implant or tissue expander alone and they require autologous reconstruction. Even under ideal circumstances, it is very difficult to achieve satisfying aesthetic reconstructions from radical mastectomies.
Mastectomy and Radiation
When treatment includes postoperative radiation, the functional deformities caused by extensive skin excision are magnified. Radiation causes scarring between skin and pectoralis major muscle as well as shrinkage of both skin and muscle; it may also have a direct causal effect on producing stiffness of the shoulder joint. Adding radiation to a modified radical mastectomy can extend the functional deficit to that of a radical mastectomy or worse. Because radiation causes persistent perivascular inflammation, it can act to damage the tissue permanently. For this reason, mastectomy flaps subjected to radiotherapy must be presumed to have poor vascularization. Therefore, much of the radiated breast skin must be discarded and replaced with autologous tissue. It is imperative to remove as much of the dyschromatic, visibly irradiated skin as possible. This discoloration provides the surgeon with visible evidence of tissue severely impacted by radiation
damage. Though this skin may survive initial re-elevation, ischemic necrosis can and often does develop many years later. Radiotherapy precludes an implant reconstruction in almost every instance since irradiated skin lacks pliability and is susceptible to late skin erosion from the underlying implant. Patients who have had augmentation can be considered for conservation therapy, which preserves the implant, but the value of this is unclear as these implants typically become firm because of the radiation, and mammography can also be impeded. Even with implants that are well positioned and intact, these factors combine to produce poor cosmesis and impaired surveillance.
damage. Though this skin may survive initial re-elevation, ischemic necrosis can and often does develop many years later. Radiotherapy precludes an implant reconstruction in almost every instance since irradiated skin lacks pliability and is susceptible to late skin erosion from the underlying implant. Patients who have had augmentation can be considered for conservation therapy, which preserves the implant, but the value of this is unclear as these implants typically become firm because of the radiation, and mammography can also be impeded. Even with implants that are well positioned and intact, these factors combine to produce poor cosmesis and impaired surveillance.
Historical Developments
The history of reconstructive breast surgery has several discrete periods, and the developments of each period have had a dramatic effect on the course of breast cancer surgery. From antiquity until recent times, healing of the mastectomy wound was the primary reconstructive concern in any extensive mastectomy procedure. Until skin grafting became tenable, radical mastectomies were allowed to heal by secondary intention. Small skin grafts were described as early as the 1870s but were not applied to the mastectomy deformity until the turn of the 20th century. Sheet skin grafting was developed in the late 1920s but was available in only a few major medical centers until after World War II. By 1950, only a few hundred surgeons were trained in sheet skin grafting, a technique that was not in general use until the Brown motorized dermatome became available. Elevation of breast skin flaps with primary closure of the mastectomy first appeared in the 1940s but was not accepted as routine until the 1960s. Breast reduction and nipple reconstruction were introduced in the late 1940s but did not gain popularity until the 1970s. The first breast reconstructions were performed by means of tubed skin flaps or silicone implants starting in the early 1960s. In Europe, Tansini reported use of the latissimus dorsi myocutaneous flap for closure of radical mastectomy defects between 1900 and 1925. Alas, myocutaneous flap procedures were all but abandoned until their renaissance in the mid-1970s. Campbell reported his use of the latissimus dorsi muscle flap for chest wall reconstruction in 1950, but his method went unnoticed for another generation. By 1975, myocutaneous flaps were being used for delayed breast and chest wall reconstructions, but myocutaneous flaps were seldom used for immediate reconstructions prior to 1990. In the present, it is difficult to imagine oncologic breast surgery without the modern adjuncts of primary closure, skin grafting, and myocutaneous flaps.
Primary Closure
Extensive skin excision has been a past hallmark of radical, modified, and even simple mastectomies. In each method, breast skin was removed in anticipation this would improve the efficacy of the procedure. This practice was so accepted that it continued for 60 years before being subjected to scientific study. The various types of mastectomy are still differentiated by factors other than skin excision. For example, the modified radical mastectomy is a radical mastectomy in which the pectoralis major muscle is preserved and the simple mastectomy is a modified radical mastectomy without axillary node dissection. Patey’s mastectomy is still considered less radical than the Halsted variant since pectoralis major muscle is preserved. However, Patey believed in extremely wide skin excision, in which only half of his mastectomies were primarily closed. Since the reports of Madden and Auchincloss in the early 1960s, primary closure was not considered harmful and thus achieved wide acceptance as the prevailing method. From the mid-1980s to early 1990s, extensive skin excision remained the standard of practice in mastectomy because of lingering suspicions that limited skin excision might be less effective in curing cancer, even though this belief had no scientific basis in fact. It will be interesting to see if our colleagues 50 years from now are as puzzled about our current practices as we are about the total breast skin excisions of the turn of the 20th century.
Primary closure in mastectomy surgery did not develop until the mid-20th century. The Halsted mastectomy, like other radical mastectomies in the early part of the 20th century, was rarely closed primarily. This procedure included resection of skin and breast tissue in a three-dimensional block (Fig. 4). This near-total removal of the breast skin was routinely used until the 1940s because elevation of breast skin flaps was
believed to interfere with tumor resection by either spreading breast cancer or by leaving it within the tissues of the breast skin. At the time, it was presumed that the breast cancer permeated the entire breast skin, not just the part of the skin that was adjacent to the tumor. When total excision of the breast skin was eventually abandoned, it was replaced with such extreme resections of the skin in the modified radical mastectomy that primary closure remained the exception until the late 1960s.
believed to interfere with tumor resection by either spreading breast cancer or by leaving it within the tissues of the breast skin. At the time, it was presumed that the breast cancer permeated the entire breast skin, not just the part of the skin that was adjacent to the tumor. When total excision of the breast skin was eventually abandoned, it was replaced with such extreme resections of the skin in the modified radical mastectomy that primary closure remained the exception until the late 1960s.
Skin Grafting
Primary healing of the radical mastectomy was rarely considered until sheet skin grafts became available in the 1920s. Until that time, very small, postage stamp sized grafts were used. Beginning in 1870, small split-thickness grafts and full-thickness grafts were used on the face. Most of these grafts were small since dermatomes had not yet been developed which would allow harvesting of larger grafts. In the 1920s, John Staige Davis popularized pinch grafts, which were harvested by shaving a “divot” of skin with a flat knife. This graft was a full-thickness graft in the center and a partial-thickness graft in the periphery to enhance the take of the graft. Even though these small grafts were available at the turn of the century, most mastectomies were nonetheless allowed to heal secondarily by scar epithelium.
Beginning in the late 1920s, Blair and Brown in the United States and Humby and Braithwaite in England popularized sheet skin grafts, which were taken with large hand-held knives. These knives were variations on the straight razors of the day and required such dexterity that fewer than a hundred surgeons were trained in these techniques by the start of World War II. Most of these surgeons were those general surgeons who sought additional training in plastic surgery. In the late 1930s, calibrated dermatomes, such as the Padgett and Reese, were introduced. The motorized Brown dermatome was not developed until after World War II when it was conceived by Dr. Brown during his wartime imprisonment in Bataan. Even in the 1950s, only a few hundred surgeons in the world attempted skin grafting procedures, and very few of these were on mastectomy patients (Fig. 5).
Early Flaps
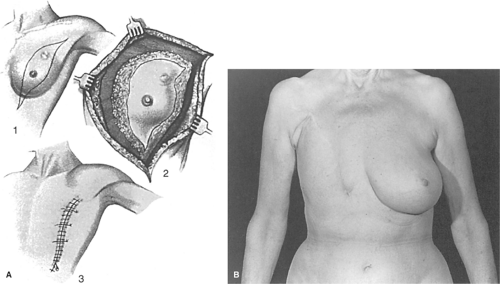
In 1896 the latissimus dorsi flap was described by Iginio Tansini, a professor of surgery at the University of Pavia in Italy. In the early part of the 19th century, the breast was removed without elevation of skin flaps, which left a large circular defect taking a number of months to heal. The Tansini method used the same extirpative technique as the Halsted radical mastectomy, but the latissimus dorsi myocutaneous flap was transposed anteriorly to achieve wound closure. The purpose of the latissimus myocutaneous flap was to replace the pectoralis major and to provide skin from the back to close the breast wound primarily. Tansini’s early 20th century description of the latissimus dorsi myocutaneous flap is virtually identical to that described independently by Olivari, McCraw, and Muhlbauer around the late 1970s. The Tansini method was the prevailing technique of mastectomy in Europe until about 1920 when Halsted made a grand tour of the European medical centers. Halsted said “Beware of the man with the plastic operation” because he thought that the latissimus flap was both “unnecessary and hazardous.” At a time when intravenous fluids, transfusions, and anesthetics were in their early development, Halsted’s statement was probably a reasonable one. Nevertheless, it sets back the legitimate use of myocutaneous flaps for breast or chest wall reconstruction for perhaps 50 years. Between 1920 and 1974, Tansini’s procedure was abandoned and myocutaneous flaps
were not used for immediate reconstruction of the breast until after 1980.
were not used for immediate reconstruction of the breast until after 1980.
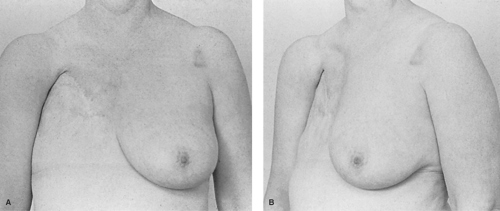 Fig. 5. A and B: Example of a skin-grafted radical mastectomy done in 1978. The sheet skin graft was a major advance in healing the mastectomy wound. |
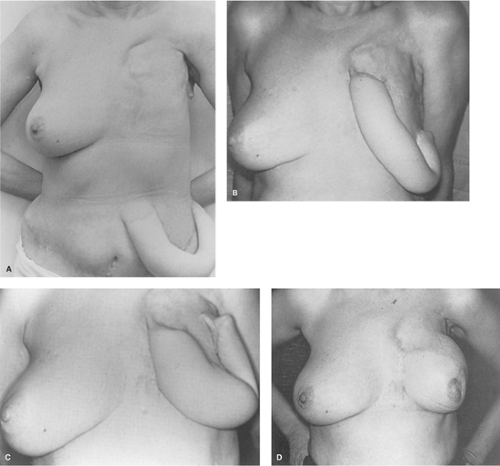
Because of the notable successes of Sir Harold Gillies in repairing World War I maxillofacial injuries, the tubed skin flap reconstructions he advocated became the dominant method of flap reconstruction until myocutaneous flaps were reintroduced in the mid-1970s. Tubed flaps were not applicable to immediate breast reconstruction because the transfer of the tube of skin from the abdomen to the breast consumed a period of at least a year and more than a dozen operations. Tubed flaps were impractical for use in treating radiation injuries since they did not carry an independent blood supply to the damaged area. Until the introduction of myocutaneous flaps, flap resurfacing of radiation wounds was seldom attempted because of high morbidity and frequent recurrence of wounds surrounding the flap. Even as late as the 1970s, tubed flaps were still being used for elective breast reconstruction (Fig. 6A,B,C,D).
Silicone Breast Implants
The introduction of silicone breast implants by Cronin and Gerow in 1964 was quickly followed by implant popularization led by a number of surgeons over the following 20 years. Their pioneering surgical work was conducted in collaboration with the Dow Chemical Company using polymers constructed from silicone.
Compared with previously available human breast implants, which were made of polyurethane foam, paraffin, or allogenic material, each of which had less than ideal characteristics, the silicone gel implants represented an historic medical breakthrough. This development also initiated the development of a completely new group of medical products derived from silicone, including silicone tubes, lubricants, and linings, which are relatively biologically inert. By the late 1960s, silicone gel implants had begun to find their way into the field of breast reconstruction. Early experience with these implants was not good because of fibrous encapsulation of the implant which occurred beneath the thin mastectomy skin flaps. The better soft tissue coverage of subpectoral placement improved results, just as the even better cover of autologous flaps improved it further. Over time, it became apparent that silicone implants were not as biologically neutral as had been hoped, and many local wound problems such as scarring, low-grade infection, and implant intolerance became causes for concern. Eventually, many of the early implant breast reconstructions failed because distortion produced an appearance known as the half-grapefruit shape, which accurately described the elevated, rounded, and firm implant (Fig. 7).