Fig. 29.1.
Fibrocystic change. (A) Cystically dilated ducts and (B) apocrine metaplasia are characteristic.
♦
Type 1 cysts are typically lined by metaplastic apocrine cells and type 2 cysts by flattened epithelium
♦
May have some epithelial hyperplasia in the spectrum of ductal intraepithelial neoplasia (low-risk ductal intraepithelial neoplasia (DIN) – see discussion later); this feature should be noted separately from FCC
Differential Diagnosis
♦
Lymphocytic mastitis:
Dense lymphocytic infiltrate around lobules and vessels with or without atypical stromal cells
Microcalcifications
♦
May be associated with benign or malignant changes
♦
Different patterns seen radiologically can be classified in the American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) as typically benign, intermediate concern, or higher probability of malignancy, but no pattern is pathognomonic
♦
Within benign breast disease:
Large, smooth, and diffuse calcifications are generally associated with benign lesions
Popcorn, milk of calcium, and teacup calcifications are a few benign patterns
♦
Within malignant breast disease:
Malignant calcifications generally show clustering and linear branching
Microcalcifications are present in almost all comedocarcinomas and about one-half of cribriform (low-grade) types
Accordingly, mammographic estimation of the extent of DIN is better for higher-grade lesions
Pleomorphic, linear, and branching patterns are associated with high-grade DIN and granular, amorphous, or rod-shaped calcifications with lower grades
♦
Calcium phosphate:
Most common form (90%)
Easily visible on hematoxylin and eosin (H&E)
Present in benign and malignant lesions
♦
Calcium oxalate:
May be difficult to see on H&E
Broken glass appearance
Requires polarized light
Usually associated with benign lesions (particularly in ducts with apocrine metaplasia)
♦
Liesegang rings:
Laminated inclusions containing calcium, iron, silicone, and sulfur
Mucocele-Like Lesion
Clinical
♦
Uncommon lesion with no distinctive clinical pattern; microscopic diagnosis
Microscopic
♦
Single or multiloculated cyst with extravasated mucin
♦
The epithelium may be in detached strips; occasionally papillary fragments
♦
The epithelium is cytologically benign
♦
Myoepithelial cells either adhere to the detached epithelial strips or line the cyst wall
♦
Cysts have fibrous wall with mild chronic inflammation
Differential Diagnosis
♦
Mucin-producing DIN 1–3:
Atypical cells in the form of arcades, cribriform, or micropapillary formations
♦
Mucinous carcinoma:
Larger cells with more cytologic atypia; more frequently papillary; cell clusters in lakes of mucin
♦
Intraductal papillary carcinoma with mucin production
♦
Infiltrating ductal carcinoma with mucin production
Juvenile Hypertrophy
Clinical
♦
Excessive and persistent enlargement of one or both breasts in young girls
♦
Aged 11–14 years
♦
Usually coincides with menarche, but may precede it
Macroscopic
♦
Appears identical to surrounding breast tissue
Microscopic
♦
Uniform but exaggerated proliferation of normal lobules and ductal structures
♦
May have occasional proliferation of ductules similar to gynecomastia
♦
Densely collagenous stroma is common
♦
Stroma may show pseudoangiomatous hyperplasia
Differential Diagnosis
♦
Fibroadenoma :
Better circumscribed grossly (shells out) and often loosely cellular stroma with an intracanalicular or pericanalicular growth pattern microscopically in contrast to the generally dense collagenous stroma of juvenile hypertrophy (JH)
♦
Normal breast tissue:
Clinical history may be necessary to distinguish juvenile hypertrophy from normal breast tissue
Gynecomastia
♦
Gynecomastia is unique to the male breast
♦
Other breast lesions/neoplasms may be found in male patients less commonly than in female patients
♦
Lesions generally felt to be more common in male patients include myofibroblastoma (see mesenchymal lesions)
Clinical
♦
Unilateral or bilateral (equal or unequal) breast enlargement
♦
Most frequent in adolescents and elderly men
♦
Etiology is either endogenous hormonal imbalance (puberty, senescence, hypogonadism, liver failure) or exogenous (drugs, chemotherapy)
Prognostic Significance
♦
Usually physiologic if in teens/elderly
♦
Otherwise requires evaluation of medical status/drug history
♦
Not a premalignant condition
Macroscopic
♦
Rubbery gray/white tissue, variable, and well-defined
Microscopic
♦
Florid type:
Increased ducts with florid epithelial proliferation, cellular periductal stroma (often myxedematous), and adipose tissue
♦
Fibrous type:
Dilated ducts, mild/moderate epithelial proliferation, hypocellular fibrous stroma, and no adipose tissue (Fig. 29.2)
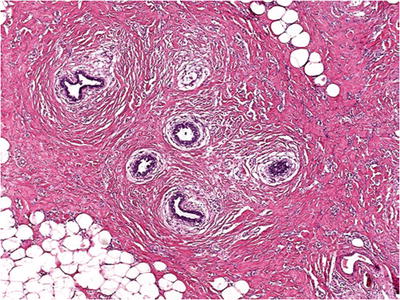
Fig. 29.2.
Gynecomastia . Ductules with a cuff of myxedematous stroma proliferate in a dense collagenous stroma with pseudoangiomatous stromal hyperplasia (PASH) in the backgound.
♦
Hyperplasia is “gynecomastoid”: angulated epithelial tufts with the smallest cells at apex overlying a stratified epithelial cell layer
♦
Myoepithelial layer preserved
♦
Apocrine and squamous metaplasia may occur
♦
Lobules are identified in 6% of cases
♦
Atypical intraductal hyperplasia is occasionally found
Differential Diagnosis
♦
Not a difficult diagnosis when you know that the sample is from a male patient
Metaplastic Changes
♦
Replacement of one cell type (mostly epithelium) with another mature cell type
Apocrine Metaplasia
♦
Cells with granular eosinophilic or foamy cytoplasm, round nuclei, and usually prominent nucleoli (Fig. 29.1B)
♦
Sometimes shows decapitation secretion or coarse hyaline granules
♦
Frequently occurs in fibrocystic change
♦
Papillary morphology common in cysts; papillae with fibrovascular cores
Differential Diagnosis
♦
Apocrine hyperplasia :
Diagnosed when there is more than the normal one- or two-cell thickness
♦
Atypical apocrine metaplasia or hyperplasia:
Nuclei show a threefold variation in size
♦
Apocrine DIN (DCIS):
Atypia with luminal necrosis
Clear Cell Metaplasia
♦
Cytoplasm clear or vacuolated, rather than granular and eosinophilic (Fig. 29.3)
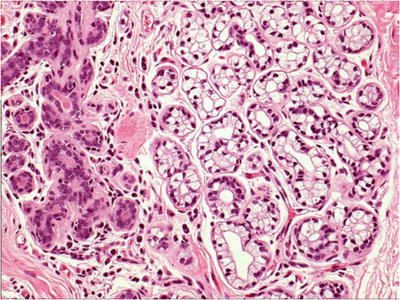

Fig. 29.3.
Clear cell metaplasia. The cells in the lobule on the right side of the image show cytoplasmic clearing in contrast to the granular eosinophilic cytoplasm on the left.
♦
May show PAS-positive globules in cytoplasm
♦
No association with clear cell carcinoma
Squamous Metaplasia
♦
Associated with the following:
Infarcted papilloma: may follow fine-needle aspiration
Phyllodes tumor
Syringomatous adenoma
Ducts associated with periareolar abscess
May focally line a biopsy cavity
Mucinous Metaplasia
♦
Relatively uncommon
♦
Typically affects normal isolated lobule
♦
May occur focally in papillomas
♦
No known preneoplastic potential
Lactational Change (Lactational Metaplasia)
Clinical
♦
Usually reproductive-age women with recent history of pregnancy
♦
Rarely in postmenopausal women, possibly drug-related (digitalis, neuroleptics); male patients on stilbestrol
♦
May present as a mass during pregnancy or at postpartum examination
Macroscopic
♦
Sharply circumscribed if involving a preexisting tubular adenoma (lactating adenoma), usually <5 cm
♦
Soft texture
Microscopic
♦
Lobules expanded with diminished stroma; no epithelial proliferation
♦
Secretory pattern:
Eosinophilic material in lumen; cells with vacuolated cytoplasm
♦
Regressive pattern:
Less secretion; dilated acini with hobnail hyperchromatic nuclei
Differential Diagnosis
♦
May be mistaken for malignancy on fine-needle aspirate (FNA): look for history, foamy background to smear, and myoepithelial cells
Adenosis
♦
Lobular expansion with increased ductules/acini but no epithelial proliferation (Fig. 29.4)
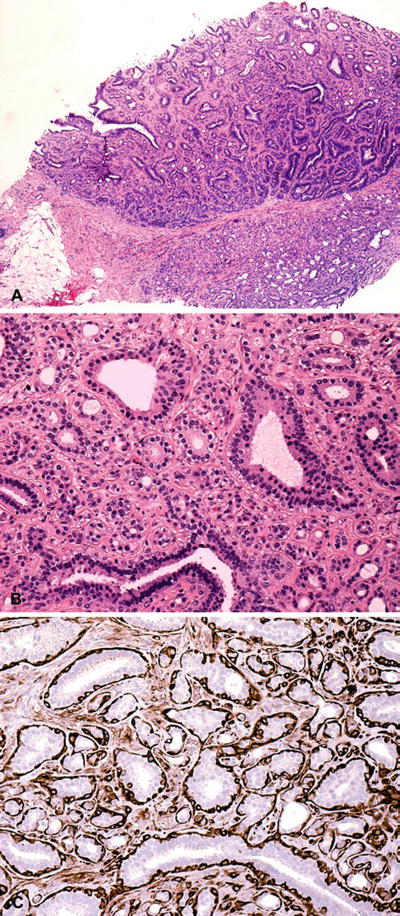

Fig. 29.4.
Nodular adenosis. (A) Some cases of adenosis may present as a distinct nodule. (B) On higher magnification, there is lobular expansion with increased ductules/acini but no epithelial proliferation. (C) Calponin highlights preservation of the myoepithelial cell layer.
♦
Various types: sclerosing, microglandular, blunt duct, adenomyoepithelial, tubular, secretory, and apocrine
Sclerosing Adenosis
Clinical
♦
Relatively common, often bilateral, lesion
♦
Occasionally forms a palpable mass <2 cm (nodular sclerosing adenosis) in size
♦
Usually a microscopic finding
♦
May be associated with increased risk of carcinoma, especially in the presence of atypia
Macroscopic
♦
Cannot be distinguished grossly from fibrocystic change
Microscopic
♦
Low power retention of lobulocentric configuration is key to diagnosis (Fig. 29.5)
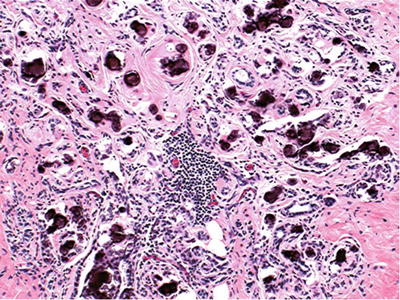

Fig. 29.5.
Sclerosing adenosis retains a lobulated configuration; microcalcification may be abundant.
♦
Fibrosis may distort or obliterate lumina and make myoepithelial cells prominent
Immunohistochemistry
♦
Smooth muscle actin (SMA) will stain myoepithelial cells, which are preserved in sclerosing adenosis
Differential Diagnosis
♦
Atypical apocrine adenosis:
Shows involvement of lobules by atypical cells with apocrine features
♦
Invasive carcinoma:
No myoepithelial cell layer and no lobulocentric pattern
Microglandular Adenosis
Clinical
♦
A rare lesion, presenting as an ill-defined palpable mass; most common among women in their fourth and fifth decades
♦
Usually 3–4 cm in size
Macroscopic
♦
No distinguishing features; variably dense, rubbery, fibrous tissue
Microscopic
♦
Proliferation of rounded, duct-like structures containing PAS-positive eosinophilic (colloid-like) secretory material in a fibrocollagenous stroma (Fig. 29.6)
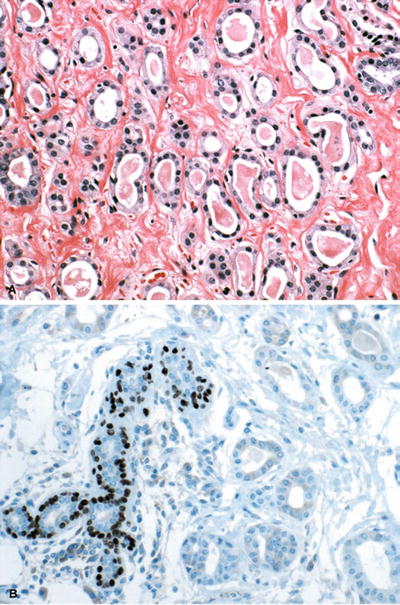

Fig. 29.6.
Microglandular adenosis . (A) Proliferating duct-like structures with luminal secretory material infiltrate in a fibrocollagenous stroma. (B) Although p63 stain demonstrates lack of a myoepithelial cell layer, the lesion is benign.
♦
Stroma varies from densely collagenous to loose and paucicellular
♦
More infiltrative growth compared to other types of adenosis
♦
Single epithelial cell layer, often with clear cytoplasm and no myoepithelial cell layer
Immunohistochemistry
♦
Epithelium of microglandular adenosis (MGA ) is positive for both S-100 and cytokeratin (CK) but negative for ER and PR
♦
No myoepithelial cell layer identified by various myoepithelial cell markers
♦
Thickened basement membrane highlighted by laminin and collagen IV
Ultrastructure
♦
Preservation of basement membrane, which is often multilayered
♦
Absence of a myoepithelial cell layer
Differential Diagnosis (See Table 29.1)
♦
Invasive tubular carcinoma:
Fibroblastic rather than fibrocollagenous stroma of MGA
Angulated glands rather than round
No luminal secretions
Eosinophilic rather than clear cytoplasm in lining cells
Apocrine snouting rather than truncated luminal cell border
No basement membrane
Pitfall: MGA may also extend into fat
♦
Atypical MGA:
Some cases of MGA show cytologic atypia and mitotic figures; regarded as intermediate stage between MGA and carcinoma
Carcinomas may arise in MGA; most retain S-100 positivity and are ER and PgR negative
♦
Tubular adenosis:
Has a myoepithelial cell layer on immunostaining for actin
Table 29.1.
Dis ting uishing Features of Tubular Carcinoma , Sclerosing Adenosis , and Microglandular Adenosis
Tubular carcinoma | Sclerosing adenosis | Microglandular adenosis | |
|---|---|---|---|
Shape of lesion | Stellate, irregular | Lobulated | Irregular |
Shape of glands | Angulated | Round to oval | Round |
Luminal contents | Sometimes | Rarely | Frequent, “colloid” |
Cell layers | 1 (Epithelial) | 2 (Epithelial and myoepithelial) | 1 (Epithelial) |
Cell luminal surface | Snouting | Occasional snouting | Smooth |
Cytoplasm | Eosinophilic | Inconspicuous | Clear or eosinophilic |
Stroma | Desmoplastic | Around lobules | Dense collagenous |
Basement membrane | Usually none | Present | Prominent, multilayered |
Complex Sclerosing Lesion/Radial Scar
Clinical
♦
Middle-aged to elderly women
♦
Frequently multiple and bilateral incidental microscopic findings
Radiology
♦
May form a stellate/spiculated mass on mammogram, described as suspicious or highly suggestive of malignancy
♦
Rarely associated with calcification
Macroscopic
♦
A minority may form a palpable mass indistinguishable from invasive carcinoma grossly
Microscopic
♦
Central fibroelastotic or fibrocollagenous scar
♦
Stellate arrangement of ducts; zonal pattern may be obscured if only part of the lesion is sampled or in core biopsy (Fig. 29.7)
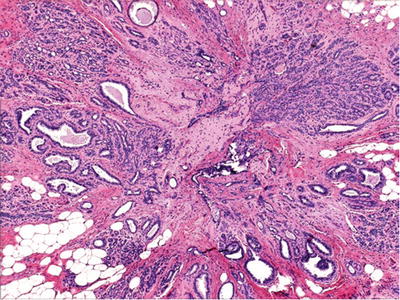

Fig. 29.7.
Radial scar is characterized by a central fibroelastotic or fibrocollagenous scar surrounded by a stellate arrangement of ducts.
♦
Maximum epithelial proliferation is at periphery
♦
Thirty percent have some atypical hyperplasia or carcinoma; however, complex sclerosing lesion/radial scar (CSL/RS) per se is not an independent risk factor for carcinoma
Differential Diagnosis
♦
Tubular carcinoma:
Distinction may be impossible on needle biopsy
CSL/RS shows preservation of myoepithelial layer on immunostaining
Duct Ectasia (Periductal Mastitis)
Clinical
♦
Majority of cases are subclinical
♦
Patients may have pain and tenderness around the nipple or chronic nipple discharge or distortion
Macroscopic
♦
Periareolar (large) ducts affected
♦
Dilated ducts with yellow material in lumen, mimicking comedocarcinoma
Microscopic
♦
Acute stages rarely seen; acute inflammation around and in duct
♦
Fibrosis leads to duct distortion
♦
Periductal lymphoplasmacytic infiltrate and pigmented histiocytes
♦
Foam cells in epithelium and lumen
♦
Duct may be obliterated by process; “ectasia” a misnomer at this stage
Collagenous Spherulosis
♦
Incidental microscopic finding; the importance is in differential diagnosis
Microscopic
♦
Spheres of eosinophilic material (20–100 mm) surrounded by myoepithelial cells, in duct lumina or TDLU (terminal duct-lobular unit) (Fig. 29.8)
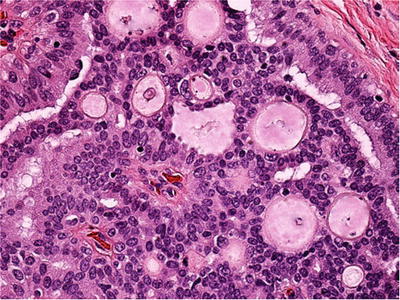

Fig. 29.8.
Collagenous spherulosis . Spheres of eosinophilic material with a peripheral “cuticle” are surrounded by myoepithelial cells.
♦
Material may be fibrillar, asteroid, or dense and amorphous
♦
Eosinophilic “cuticle” at periphery characteristic
♦
Spaces may contain mucinous, flocculent basophilic material: “mucinous spherulosis”
♦
The epithelial cell population around collagenous spherulosis may be benign, atypical, or malignant; judge separately from collagenous spherulosis
Immunohistochemistry
♦
Spheres positive for collagen IV
♦
Surrounding myoepithelial cells positive for p63 and calponin
Differential Diagnosis
♦
Ductal intraepithelial neoplasia 1c (ductal carcinoma in situ, low grade), cribriform type
May also show basophilic material in spaces; no myoepithelial cells around cribriform spaces
♦
Adenoid cystic carcinoma
More extensive and with more cellular atypia and usually forms a mass
♦
Lobular neoplasia with signet-ring cells
Look for other cells with intracytoplasmic lumina
Mastitis
Acute Mastitis/Periareolar Abscess
Clinical
♦
Reproductive years
♦
Crack in the skin of the nipple, frequently in nursing women, and allows bacterial entry
♦
Congenital abnormality/inversion of the nipple increases risk
♦
Rarely a surgical specimen
Microscopic
♦
Squamous metaplasia of large ducts
♦
Thick-walled abscess cavity in untreated cases
♦
Staph. aureus and anaerobes are the usual organisms
Granulomatous Mastitis
♦
Idiopathic accounts for most cases in the West (Fig. 29.9)
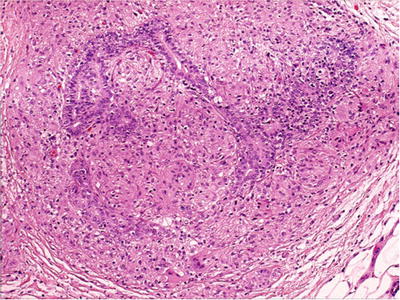

Fig. 29.9.
Granulomatous mastitis . Most cases are idiopathic.
♦
Other causes include the following:
Tuberculosis: necrotizing granulomas; isolation of M. tuberculosis required for diagnosis
Fungi and protozoa
Reaction to duct rupture
Reaction to carcinoma
Sarcoid: usually in context of established disease elsewhere; rule out other causes
Wegener granulomatosis
Idiopathic Granulomatous Mastitis
Clinical
♦
Usually reproductive age, but wide range
♦
Tender, palpable nodule; may be bilateral
♦
Etiology unknown; epithelial damage may be a primary event
Microscopic
♦
Granulomatous inflammation centered on lobules
♦
Mixed inflammatory cell infiltrate
♦
Diagnosis based on excluding other causes of granulomatous inflammation
Silicone Reaction
Clinical
♦
Leakage of silicone from implants/implant rupture is the usual cause
♦
Injection of silicone for augmentation no longer performed
♦
Reaction may be to additives and/or silicone
Microscopic
♦
Empty spaces or spaces with refractile foreign material
♦
Histiocytic or foreign body giant cell reaction with fibrosis
♦
Similar changes frequent in regional nodes
Differential Diagnosis
♦
Fat necrosis
♦
Metastatic mucinous carcinoma (lymph nodes or bone marrow)
Lymphocytic Mastitis (Diabetic Mastopathy, Fibrous Mastopathy)
Clinical
♦
Painless mass
♦
Wide age range of 24–72 years; occasional cases in male patients
♦
Often associated with type I diabetes mellitus or other human leukocyte antigen (HLA)-associated autoimmune disease
Macroscopic
♦
Dense, rubbery, fibrous tissue
Microscopic
♦
Extensive stromal collagen with atrophy of acini (Fig. 29.10)
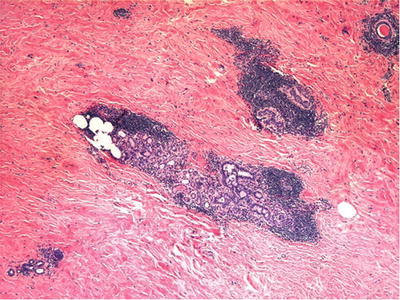

Fig. 29.10.
Lymphocytic mastitis typically has extensive stromal collagen with atrophy of acini and a dense lymphocytic infiltrate surrounding residual lobules.
♦
Dense lymphocytic infiltrate surrounds residual lobules – may have germinal centers; however, association with mucosa-associated lymphoid tissue (MALT) lymphoma is unlikely
♦
Lymphocytic perivasculitis or vasculitis is a constant feature
♦
Prominent epithelioid stromal cells, some binucleate
Immunohistochemistry
♦
Stromal cells negative for keratin; may be KP1-positive
♦
Lymphocytes are B-cells
Differential Diagnosis
♦
Infiltrating carcinoma:
Hypocellularity and atypia of stromal cells may suggest lobular carcinoma
♦
Fibrocystic change:
Inflammation in fibrocystic change (FCC) is more periductal than perilobular
Amyloidosis
Clinical
♦
May occur as an amyloid tumor (elderly women) or microscopic finding
♦
Patients have systemic disease (e.g., rheumatoid arthritis or amyloidosis)
Macroscopic
♦
Nodule with granular or waxy cut surface
Microscopic
♦
Amorphous eosinophilic material with giant cell reaction
♦
Vascular and adipose tissue deposition in non-nodular forms
♦
Pink on Congo red stain, apple green birefringence on polarizing
Differential Diagnosis
♦
May be mistaken for carcinoma clinically and grossly
♦
Microscopically, elastosis may resemble amyloid
♦
Areas of collagen in lymphocytic mastitis may resemble amyloid
Fat Necrosis
Clinical
♦
Usually an incidental microscopic finding, occasionally a palpable mass
♦
Although most cases are believed to be due to trauma, history of trauma or radiotherapy is elicited in less than half
♦
May be accompanied by pain/tenderness/bruising/skin retraction
Macroscopic
♦
Well-defined, firm area usually <2 cm in size
♦
Early lesions show hemorrhage
♦
Late lesions show scar with cyst formation
Microscopic
♦
Anucleate fat cells surrounded by foamy histiocytes (Fig. 29.11)
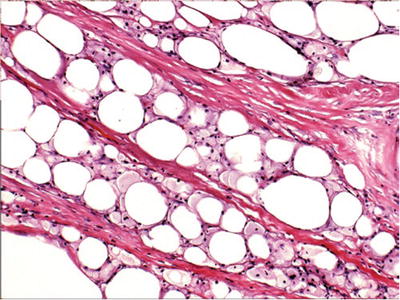

Fig. 29.11.
Fat necrosis is characterized by anucleate fat cells surrounded by foamy histiocytes. Fibrosis and calcification may develop in later lesions.
♦
Fibrosis and calcification in later lesions
Benign Neoplasms
Benign Epithelial Neoplasms
Adenomas and Variants
♦
Ductal adenoma – see sclerosing papilloma
♦
Syringomatous adenoma – see “Nipple Lesions.”
♦
Nipple duct adenoma – see “Nipple Lesions.”
Tubular Adenoma
♦
Benign, well-circumscribed proliferation of tubular structures, lined by epithelium and myoepithelium
Clinical
♦
90% of patients <40 years; rare in men
♦
Mobile mass, may be tender; frequently discovered in pregnancy
Macroscopic
♦
Solid, firm, tan-yellow nodule >1 cm in size
ZMicroscopic
♦
Encapsulated or well defined
♦
Closely packed tubules (Fig. 29.12)
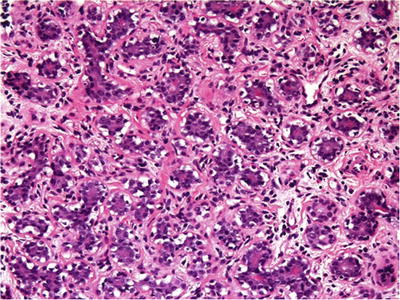

Fig. 29.12.
Tubular adenoma presents as an encapsulated or well-defined mass composed of closely packed tubules in a hypocellular stroma with scant lymphocytes.
♦
Hypocellular stroma with scanty lymphocytes
♦
Epithelium may show mitotic figures, apocrine metaplasia, and lactational or secretory changes; atypia is rare
Differential Diagnosis
♦
Fibroadenoma has a neoplastic stromal component: lesions with features of both may occur (combined tubular and fibroadenomas)
♦
Nodular adenosis: less well-defined/not encapsulated
Apocrine Adenoma
♦
Rare adenomas with complete replacement of epithelial cells by metaplastic apocrine cells
♦
Sharply demarcated from the surrounding breast tissue
♦
Compact proliferation of ductules lined by a single layer of epithelial cells overlying myoepithelial cells
Lactating Adenoma
Clinical
♦
Women in reproductive age group; discovered when pregnant or nursing
Macroscopic
♦
Well circumscribed, yellow, and soft
Microscopic
♦
May vary according to time of removal
♦
Sharply circumscribed, lobular arrangement maintained (Fig. 29.13):
Pregnancy: secretory material in lumen and vacuolated cytoplasm
Postpartum: marked distention, hobnail cells, and vacuolated cytoplasm
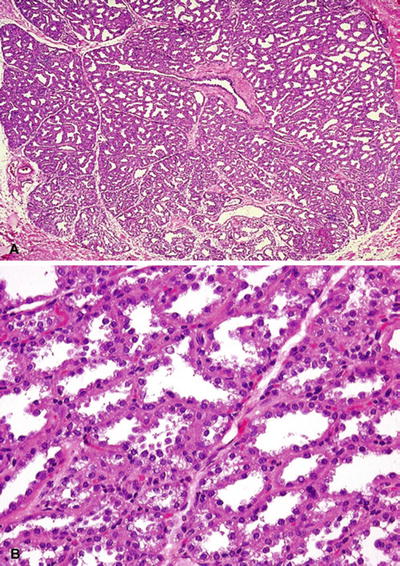
Fig. 29.13.
Lactating adenoma . (A) A well-circumscribed mass in women who are pregnant or nursing is typical. (B) On higher magnification, there is lactational-type change with vacuolization and hobnail cells. With permission from Tavassoli FA and Eusebi V. Tumors of the mammary gland, series 4. Armed Forces Institute of Pathology Atlas of Tumor Pathology 2009. © American Registry of Pathology
♦
Infarction may be seen
♦
Attenuated myoepithelial cells become inconspicuous
Differential Diagnosis
♦
Lactational change: more diffuse process and similar cytologically
♦
Carcinoma: pitfall on FNA, look for bubbly background, myoepithelial cells
Pleomorphic Adenoma (Benign Mixed Tumor)
Clinical
♦
Rare
♦
Usually in elderly women but also reported in teenagers and men
Macroscopic
♦
Well circumscribed, lobulated, myxoid, or chondroid appearance
Microscopic
♦
Identical to those of the salivary glands morphologically
♦
Epithelial/myoepithelial proliferation in a myxochondroid background with focal ossification
Immunohistochemistry
♦
Cytokeratin: positive in epithelial cells
♦
SMA/S-100/p63/CD10/calponin: positive in myoepithelial cells
♦
Glial fibrillary acidic protein (GFAP): In breast, myoepithelial cells are less commonly positive than in salivary gland lesions
Hamartoma
Clinical
♦
Wide age range, usually 30s or 40s
♦
Detected on screening; well-delineated density on mammograms
Macroscopic
♦
Oval, usually approximately 3 cm in size; rubbery consistency
Microscopic
♦
Well circumscribed, often encapsulated
♦
Variable quantities of fibroadipose or glandular tissue, showing alterations seen in the normal breast (fibrocystic change, apocrine metaplasia, adenosis) (Fig. 29.14)
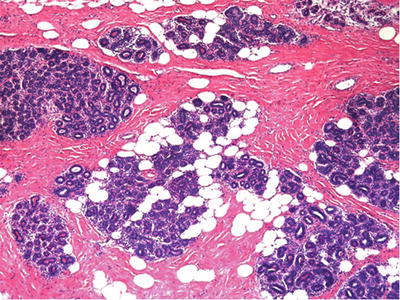
Fig. 29.14.
Mammary hamartoma presents as a mass composed of variable quantities of otherwise unremarkable fibroadipose and glandular breast tissue which by definition must be surrounded by a capsule, even if it is only a delicate one.
Differential Diagnosis
♦
Normal breast
May not be possible to distinguish unless sectioned to show capsule
Benign Mesenchymal Tumors
Fibromatosis
Clinical
♦
Less common in the breast than other sites
♦
Female patients, median age = 25; occasionally bilateral
♦
Mass and skin retraction mimics carcinoma
♦
Stellate pattern on mammography also suggests carcinoma
Prognostic Significance
♦
Higher risk of recurrence in the breast compared to extramammary fibromatosis; inking and extensive sampling of specimen are important to ensure adequate excision
♦
No metastatic potential
Macroscopic
♦
Ill defined, soft, gray-white lesion, 1–10 cm in size
Microscopic
♦
Irregular margin with fingers extending into breast tissue and surrounding ducts
♦
Variably cellular, with intervening collagen; spindle cells are in sheets and occasionally show a storiform pattern of growth
♦
No or mild atypia
♦
Mitoses <3/10 high power fields (HPF) with no atypical forms
♦
In younger women (childbearing age), there is usually more cellularity, atypia, and mitotic activity than in perimenopausal and postmenopausal women
♦
No calcification or necrosis; chronic inflammatory cells are frequent, especially in older patients
Immunohistochemistry
♦
Spindle cells are positive for vimentin and beta catenin; a minority for actin; negative for keratin, CD34, and S-100 protein
♦
Despite origin from breast stroma, the vast majority are negative for hormone receptors
Differential Diagnosis
♦
Low-grade fibrosarcoma:
Difficult on occasion; fibrosarcoma has more mitoses and at least some nuclear atypia
♦
Spindle cell carcinoma:
More pleomorphism and mitotic figures with keratin-positive spindle cells (high molecular weight CK)
♦
Nodular fasciitis:
Rare diagnosis in breast, but may occur on adjacent chest wall; contains occasional multinucleated cells; inflammatory infiltrate is at periphery of the lesion
Myofibroblastoma
Clinical
♦
Usually a solitary, slow-growing, painless, firm, and mobile mass
♦
Mostly occurs in older men and postmenopausal women
♦
Excision is curative
Macroscopic
♦
Nodular, well-circumscribed, but unencapsulated rubbery lesions
Microscopic
♦
Spindle cell proliferation arising from mammary stroma arranged in fascicles and interspersed by bands of dense collagen
♦
Mitoses are absent or rare
♦
Occasional cases show fat or cartilage
♦
Some breast ducts may be entrapped in the lesion
♦
Numerous mast cells
♦
Variants: cellular, infiltrating, epithelioid, deciduoid-like, lipomatous, collagenized/fibrous, myxoid, and mixed
Immunohistochemistry
♦
Typically positive for vimentin, desmin, and CD34
♦
Variable positivity for actin, CD99, and bcl-2
♦
Negative for cytokeratin, epithelial membrane antigen (EMA), S-100, homatropine methylbromide (HMB) 45, and c-kit (CD117)
♦
Often positive for estrogen receptors (ER), progesterone receptors (PgR), and androgen receptors (AR)
Ultrastructure
♦
Bundles of 6-nm-diameter myofilaments and no tonofilaments
♦
Rare pinocytotic vesicles
♦
Elongated nuclei; cytoplasm with prominent rough endoplasmic reticulum and Golgi apparatus
Cytogenetics
♦
Partial loss of 13q and 16q suggests relationship to spindle cell lipoma
Differential Diagnosis
♦
Fibromatosis:
Has a more infiltrative pattern; may be mistaken with infiltrating variant
Negative for hormone receptor immunohistochemistry
♦
Nodular fasciitis:
Stellate pattern, plumper mesenchymal cells
♦
Neurofibroma:
More angulated nuclei
♦
Neurilemmoma (schwannoma):
Antoni A and B areas, Verocay bodies
♦
Spindle cell lipoma:
Intermixed fat; may be difficult to distinguish from lipomatous variant
♦
Carcinoma (versus epithelioid or deciduoid-like variants):
Keratin positive
Granular Cell Tumor
Clinical
♦
Uncommon in the breast; wide age range (mean: 40 years); women > men
♦
More common in African Americans
♦
May be detected as stellate lesions on mammogram
♦
May be near the nipple, distorting it
♦
Cured by excision; <1% malignant
Macroscopic
♦
Frequently small (~1 cm) sharply circumscribed firm lesions
♦
May mimic carcinoma with a stellate shape and firm consistency
Microscopic
♦
Has a focally infiltrative margin in areas, despite gross appearance
♦
Sheets, nests, or cords of large, polygonal cells with granular eosinophilic cytoplasm
♦
Small eccentric nuclei, occasionally mild pleomorphism
♦
Mitoses are rare; no necrosis
♦
A highly infiltrative variant is often mistaken for carcinoma
Immunohistochemistry
♦
Diffuse positivity for S-100 protein
♦
Negative for cytokeratin, EMA, and ER/PgR
Differential Diagnosis
♦
Invasive ductal carcinoma:
Principally mimics this on gross appearance and FNA
♦
Normal nipple:
Small granular cell tumor may be difficult to see in this region
♦
Leiomyoma
May arise from the smooth muscle in the periareolar region
Neurilemmoma (Schwannoma)
Clinical
♦
Rare in the breast; men and women; wide age range
Microscopic
♦
Hypercellular (Antoni A) and hypocellular (Antoni B) areas, with Verocay bodies
♦
Identical to these tumors elsewhere
♦
Degenerative stromal and nuclear changes may occur
Lipoma
Clinical
♦
Solitary soft mass; occurs in women in their 40s and 50s
Macroscopic
♦
Soft, yellow, and well demarcated
Microscopic
♦
Mature adipose tissue; thin fibrous capsule is necessary to make the diagnosis
♦
Variants:
Prominent spindle cell component: spindle cell lipoma
Prominent vessels with fibrin thrombi: angiolipoma
Differential Diagnosis
♦
Normal breast adipose tissue
Intraepithelial Proliferations
♦
General term = mammary intraepithelial neoplasia (MIN)
♦
Divided into ductal intraepithelial neoplasia (DIN) and lobular intraepithelial neoplasia (LIN)
♦
The designation of MIN may be appropriate when an in situ proliferation has both ductal and lobular characteristics or does not quite match one or the other
Summary of Cytologic and Architectural Characteristics
♦
See microscopic description of individual categories and Table 29.2
Table 29.2.
Ordinary Ductal Hyperplasia (Low-Risk DIN) Versus Atypical Ductal Hyperplasia (DIN 1b, ≤2 mm): Distinguishing Features
Low-risk DIN | DIN 1b (≤2 mm) | |
|---|---|---|
Cellular proliferation | Two cell types (mixed pattern) | Epithelial only (monotonous pattern) |
Secondary lumina | Slit-like, irregular | Rigid “Roman bridges”, rounded |
Cell borders | Indistinct | Distinct |
Nuclei | Variable in shape | Round contour |
Nucleoli | Usually absent | Often present |
Necrosis | May be present | If present = DIN 2 or 3 |
High-molecular-weight cytokeratin | Present | Absent or significantly reduced |
Features of Ductal Proliferations
♦
Cohesive cells that display distinct cell margins when atypia develops
♦
Secondary lumen formation (rosettes)
♦
Generally, larger nuclei than lobular neoplasia
♦
Variants: stratified spindle cell, spindle cell, and apocrine
Features of Lobular Proliferations
♦
Indistinct cell borders
♦
Solid or loosely cohesive pattern of growth
♦
Intracytoplasmic lumina
♦
Generally small, uniform nuclei (variant: pleomorphic lobular has larger nuclei)
Ductal Intraepithelial Neoplasia
Clinical
♦
Discovered incidentally on biopsy specimen or in biopsies for mammographically detected microcalcifications
♦
DIN 1b and higher grades are a risk factor (marker) for ipsilateral breast cancer; half of these are invasive (see Table 29.3)
Table 29.3.
Spectrum of Preinvasive Mammary Ductal Proliferations (Ductal Intraepithelial Neoplasia, DIN)
DIN | Current designation | Significant atypia a | Necrosis | Absolute risk of invasion (%) | Molecular findings | Reexcision for positive/close margin |
|---|---|---|---|---|---|---|
Low-risk DIN | IDH | − | − or +b | 1.6–1.9 | Clonalb/MSI | No |
Flat DIN 1a | Flat epithelial atypia | − | − | Questionable, probably minimal | Clonal/LOH | No |
DIN 1b (≤2 mm) | AIDH | − | − | 5.1–12 | Clonal/LOH | Yes, if extensive (≥20 partially involved ducts) |
DIN 1c (>2 mm) | DCIS, grade 1 | − | − | 10–32 | Clonal/LOH | Yes |
DIN 2 | DCIS, grade 2 | −/+(+) | +/− | 20–75 | Clonal/LOH | Yes |
DIN 3 | DCIS, grade 3 | +++ | Usually | 20–75 | Clonal/LOH | Yes |
♦
Risk increases with increasing grade
♦
Risk for an individual patient depends on family history
♦
Adequacy of surgical margin is increasingly recognized as being of importance in determining the efficacy of breast-conserving therapy for DIN 1b and higher grades
Low-Risk DIN (Usual Ductal Hyperplasia)
Microscopic
♦
Proliferation of cells characterized by difference in cell populations (epithelial and myoepithelial cells participate, heterogeneous population), nuclear overlapping, indistinct cell borders, and irregular slit-like secondary lumina (Fig. 29.15)
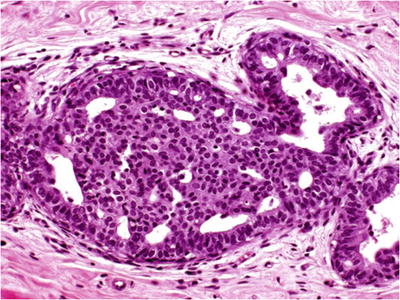

Fig. 29.15.
Low-risk DIN (intraductal hyperplasia). Prominence of peripheral irregularly sized and shaped fenestrations is characteristic. This proliferation is associated with a slightly increased risk for subsequent development of invasive carcinoma.
Immunohistochemistry
♦
Cells react with high-molecular-weight cytokeratins (CK903 and CK5/6) in a diffuse or mosaic pattern
DIN 1a (Flat DIN 1, Flat Epithelial Atypia)
Clinical
♦
Represents one of the earliest morphologically recognizable neoplastic alterations of the breast
♦
Risk for subsequent malignancy is unknown
♦
Recognition is important for early detection of intraductal neoplasia and prevention of misinterpretation as normal control in studies
♦
May explain a portion of the 20% recurrence rate of breast carcinomas excised with “negative” margins
Microscopic
♦
Replacement of native epithelial cells by one to five layers of mildly atypical cells (Fig. 29.16)
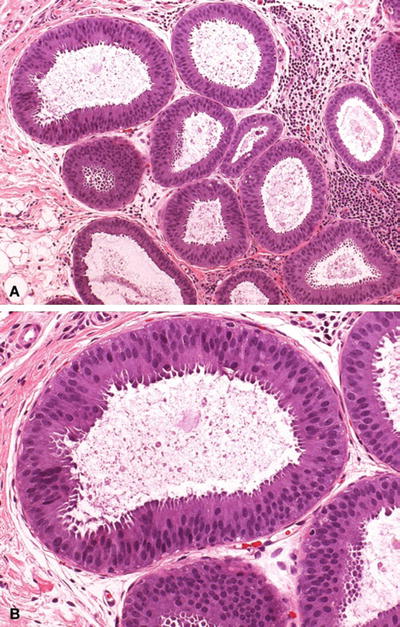

Fig. 29.16.
DIN 1a (flat epithelial atypia). (A) Distended TDLUs contain floccular secretory material. (B) Higher magnification shows the native epithelium to be replaced by one to five layers of monotonous atypical columnar cells.
♦
Monomorphous variant (so-called clinging carcinoma): frequently a single layer of abnormal cells lining a duct space
♦
A variant of flat DIN with high-grade nuclear atypia is designated as flat DIN 3 (pleomorphic variant of clinging carcinoma)
Immunohistochemistry of DIN1 (a, b, and c)
♦
Cells do not react with high-molecular-weight CK
♦
Cells react with E-cadherin
DIN 1b (≤2 mm; Atypical Ductal Hyperplasia)
Microscopic
♦
Proliferation with some cytologic (monotonous population, rounded nuclei, distinct cell borders) and architectural (rounded secondary lumina) atypia (Fig. 29.17)
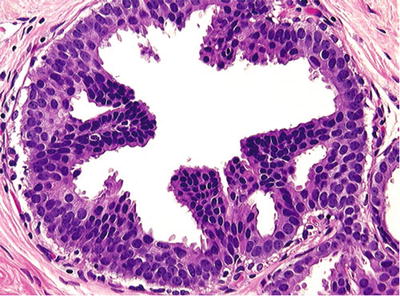

Fig. 29.17.
DIN 1b, <2 mm (atypical intraductal hyperplasia). Micropapillae composed of uniform cells partially involve the duct cross section. This limited neoplastic proliferation has a moderately increased risk for subsequent development of invasive carcinoma.
♦
Quantitatively limited: maximum complete involvement of one or more ducts not exceeding 2 mm in aggregate cross-sectional diameter
♦
Incomplete duct involvement by a process that is otherwise cytologically and architecturally similar to low-grade ductal carcinoma in situ (DCIS) also qualifies as DIN 1b
♦
DIN 1b is usually focal
DIN 1c (>2 mm; Ductal Carcinoma In Situ, Low Grade)
♦
Patterns of low-grade DCIS including cribriform and micropapillary patterns (rarely solid) without necrosis measuring greater than 2 mm in aggregate cross-sectional diameter (Fig. 29.18)
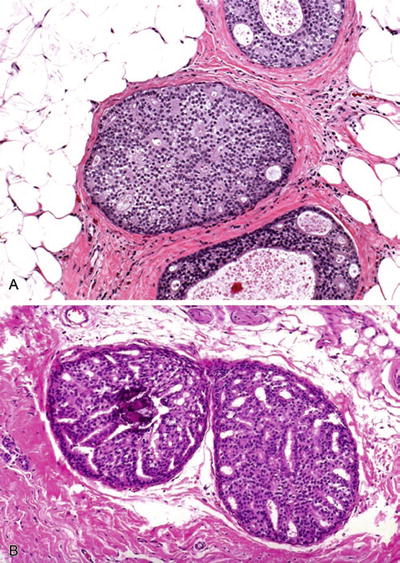

Fig. 29.18.
DIN 1c, >2 mm (DCIS, grade 1). (A) A sieve-like/cribriform proliferation of uniform cells. (B) Microcalcifications are the basis for mammographic detection of these lesions.
♦
Small monomorphic cells with round nuclei, regular chromatin pattern, and inconspicuous nucleoli
DIN 2 (Ductal Carcinoma In Situ, Intermediate Grade)
Microscopic
♦
Low-grade patterns promoted one grade by the presence of necrosis or low-grade patterns with moderate cytologic atypia
♦
Necrosis is defined as nuclear debris of five or more cells; apoptosis of individual cells or granular amorphous luminal debris should not be used to diagnose it
Immunohistochemistry
♦
Cells are negative for high-molecular-weight CK
♦
Cells are positive for E-cadherin
DIN 3 (Ductal Carcinoma In Situ, High Grade)
Macroscopic
♦
May be recognized grossly as multiple punctate yellow areas approximately 1 mm in size
♦
May be up to 8–9 cm in size, with fibrosis around ducts mimicking an invasive carcinoma
Microscopic
♦
Any case with severe cytologic atypia is grade 3
♦
Cases with moderate atypia and comedonecrosis are also grade 3
♦
Comedo DIN3/DCIS should be reserved for those with grade 3 nuclei and necrosis (Fig. 29.19)
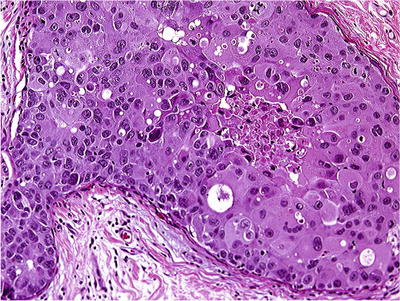

Fig. 29.19.
DIN 3 (DCIS, grade 3), comedo type. Intraluminal necrosis and cytologic atypia are present. DIN 3 is a preinvasive lesion with the highest risk for progression to an invasive carcinoma.
♦
Extensive periductal fibrosis and inflammation are common
Immunohistochemistry
♦
Cells are negative for high-molecular-weight CK in 92–94% of cases
♦
Cells are positive for E-cadherin
♦
HER2 and p53 status correlates with grade; ER/PgR usually shows inverse correlation with grade
Cytologic Variants of DIN (DCIS)
Papillary DIN (DCIS)
♦
See separate section (“Papillary Lesions”)
Apocrine DIN (DCIS)
Clinical
♦
No definitive clinical significance; importance is in differential diagnosis
♦
Apocrine cells have androgen receptors but are devoid of estrogen and progesterone receptors in at least 90% of cases
Microscopic
♦
Apocrine cytology: granular eosinophilic cytoplasm, large nuclei, and prominent nucleoli
♦
Apical snouting and cytoplasmic vacuolization variable
♦
Architectural patterns include solid or cribriform; papillary variant is not well defined
♦
Two variants are described:
Necrotic variant shows luminal necrosis and severe atypia (Fig. 29.20)
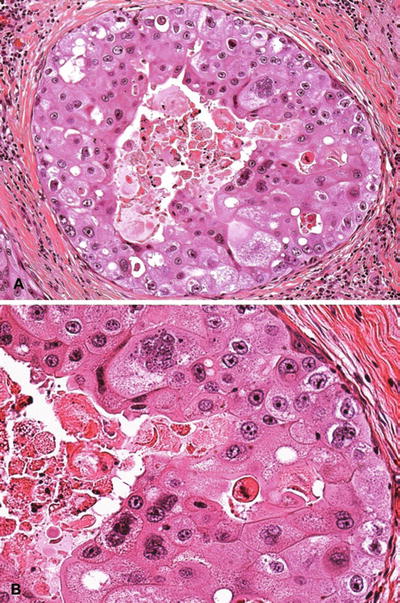
Fig. 29.20.
Apocrine DIN (DCIS). (A) Necrotic variant. (B) Higher magnification shows severe cytologic atypia.
Nonnecrotic variant has more variable atypia, usually solid or cribriform
Differential Diagnosis
♦
Apocrine metaplasia/hyperplasia:
No nuclear atypia; papillary fronds have fibrovascular cores
♦
Atypical apocrine metaplasia:
Atypia=threefold variation in nuclear size
♦
Atypical apocrine hyperplasia:
Atypia and more than two cell layers
Epithelial bridges lacking fibrovascular cores, even with bland cytology, imply a diagnosis of atypical apocrine hyperplasia
Clear Cell DIN (DCIS)
Microscopic
♦
Cells with optically clear cytoplasm and distinct cell borders
♦
May be mixed with other patterns
♦
May have associated invasive clear cell carcinoma
♦
Usually DIN 2 showing moderate nuclear atypia with or without necrosis
Signet-Ring Cell DIN (DCIS)
Microscopic
♦
Rare variant, often with cribriform architecture and composed of cells with signet-ring morphology, but positive for E-cadherin
Differential Diagnosis
♦
Intraductal papillary carcinoma:
May have a signet-ring component
♦
Mammary intraepithelial neoplasia:
Proliferation may have mixed ductal and lobular features
♦
Collagenous spherulosis:
Flattened myoepithelial cell nuclei at periphery of aggregates of collagen may mimic signet-ring cells
Secretory DIN (DCIS)
Microscopic
♦
Rare variant
♦
Dilated ducts with eosinophilic luminal secretion
♦
Epithelial tufting (papillary); cribriform growth is most usual
♦
Mitotic figures may be present
♦
Moderate nuclear atypia
♦
Cytoplasmic vacuolization
Differential Diagnosis
♦
Lactational change:
More generalized change (but not invariably); cribriform growth is not a feature
♦
Low-risk DIN with secretory change:
Heterogeneous cell population; typical architecture of low-risk DIN
high-molecular-weight keratin positive
Spindle Cell DIN (DCIS)
Microscopic
♦
Rare variant; importance lies in differential diagnosis
♦
Monotonous proliferation of elongated cells, forming solid pattern
♦
Often admixed with a relatively solid variant of cribriform DIN; this may lead to a misinterpretation of the two-cell population as low-risk DIN
♦
May show neuroendocrine differentiation
Differential Diagnosis
♦
Intraductal hyperplasia:
Has a spindle cell population, but does not display the monotony of pure spindle cell DIN (DCIS)
Irregular peripheral fenestrations as opposed to the “punched out” cribriform pattern of mixed cribriform and spindle cell DIN (DCIS)
Positive for high-molecular-weight keratins
♦
Solid papillary DIN 1c (DCIS):
May have spindle cell component
Lobular Intraepithelial Neoplasia
Clinical
♦
A pathologic diagnosis; encompasses atypical lobular hyperplasia and lobular carcinoma in situ
♦
Occurs in 0.3–3.8% of all breast biopsies
♦
Natural history difficult to determine
♦
Marks patients as being at increased risk of invasive carcinoma, both lobular and ductal, in both breasts (risk is higher in the ipsilateral breast)
♦
Patients with grade 3 lesions are at higher risk
♦
Ductal involvement is not a risk factor for recurrence
♦
The role of family history in increasing risk is not determined
Macroscopic
♦
Not detectable macroscopically
Microscopic
♦
Generally, a population of small uniform cells lacking nucleoli (Fig. 29.21)
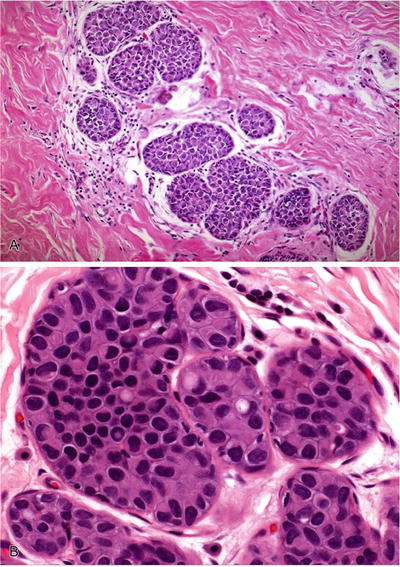

Fig. 29.21.
Lobular intraepithelial neoplasia. (A) Solid occlusive proliferation of uniform, loosely cohesive small cells. (B) Intracytoplasmic lumina are typical of LIN.
♦
Intracytoplasmic lumina are present
♦
Cell borders are indistinct; cells are frequently loosely cohesive
♦
Extension into terminal ducts is found in approximately two-thirds of cases (Fig. 29.22)
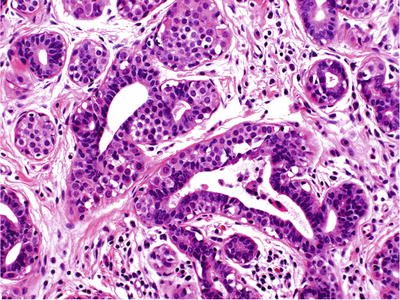

Fig. 29.22.
Pagetoid extension of neoplastic lobular cells to adjacent ducts.
♦
Necrosis and calcification denote a higher grade (Fig. 29.23)
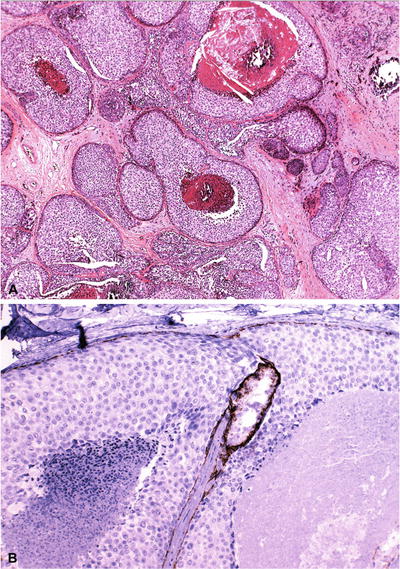

Fig. 29.23.
LIN with necrosis is considered high grade or LIN 3 (A). Although the necrosis is comedo-type – reminiscent of DIN 3 – negative E-cadherin confirms this is a lobular proliferation (B); note the positive internal control.
♦
“Clover-leaf pattern” due to unfolding of terminal duct lobular unit (TDLU)
Immunohistochemistry
♦
Cells are negative for E-cadherin
♦
Cells are positive for high-molecular-weight CK
Grading
♦
LIN grade 1 (classic LIN/ALH):
Partial or complete replacement of normal acinar epithelium; acini are not distended
♦
LIN grade 2 (classic LIN/LCIS):
Distention of some or all acini but with preservation of interlobular stroma
♦
LIN grade 3 (high-grade LIN/LCIS):
Massive distention and confluence of acini (macroacinar type)
Necrotic, pleomorphic (marked nuclear size variation), and signet-ring cell types are also grade 3
♦
For practical purposes, LIN can be designated as classic/low grade (LIN 1 and 2) or high grade (LIN 3)
Differential Diagnosis
♦
DIN 1 (atypical intraductal hyperplasia)
See cytologic features listed above
Some cases may be impossible to distinguish from DIN 1 because of overlapping morphologic and immunohistochemical features; these could be designated as MIN. For management purposes, E-cadherin-negative lesions are managed as LIN and E-cadherin-positive lesions as ductal, regardless of reactivity with CK903 or CK5/6
♦
Collagenous/mucinous spherulosis
Increased difficulty when LIN involves a preexisting benign lesion that alters architectural pattern (e.g., spherulosis, sclerosing adenosis, complex sclerosing lesions)
Microinvasive Breast Carcinoma
♦
A cluster of tumor cells breaking through the basement membrane infiltrating the periductal stroma, with or without visible continuity with that duct, the diameter of the area not exceeding 2 mm
♦
If multiple, up to three foci, each up to 1 mm in diameter
Clinical
♦
If clearly defined, cases with microinvasion can be managed as DCIS
♦
Up to 3% of cases diagnosed as DCIS reportedly have lymph node metastases. These arise from areas of invasive carcinoma not detected in the biopsied or sampled areas
Macroscopic
♦
Cannot be distinguished from intraepithelial neoplasia
Microscopic
♦
Tongue-like projection from a duct or small group of cells beside a duct with DIN1c or DIN 2-3 (DCIS) (Fig. 29.24)
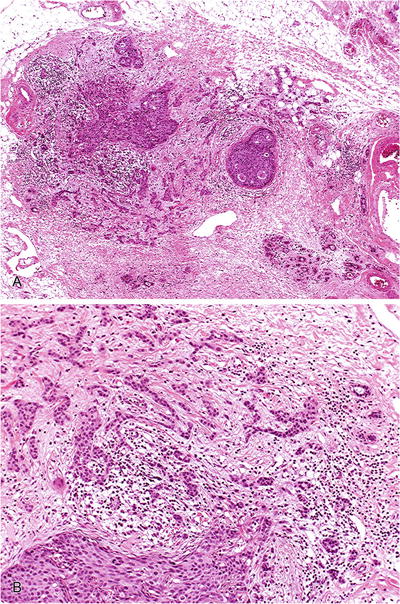

Fig. 29.24.
Early infiltrating ductal carcinoma. (A) Low-power magnification shows microinvasion. (B) At higher magnification, there is infiltration of stroma in irregular angulated clusters.
Stroma frequently fibroblastic and myxoid
Infiltrating Carcinoma
Reporting of Infiltrating Breast Carcinoma
♦
Data elements required in the pathology report include the following:
Type of specimen (surgical procedure)
Specimen laterality
Tumor histologic type
Size of tumor (at least maximum dimension in cm)
Grade of tumor (Nottingham; see below)
Tumor focality (single or multifocal)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


