CHAPTER 19 Brain stem
The brain stem consists of the medulla oblongata, pons and midbrain. It is situated in the posterior cranial fossa, and its ventral surface lies on the clivus. It contains numerous intrinsic neuronal cell bodies and their processes, some of which are the brain stem homologues of spinal cell groups. Some brain stem cell groups are the nuclei of cranial nerves III–XII: they are concerned with the sensory, motor and autonomic innervation of the head and neck. Other autonomic fibres that arise from the brain stem are distributed more widely via the vagus nerve. The brain stem also contains a complex and sometimes ill-defined network of neurones, the reticular formation, that extends throughout its length, and is continuous caudally with its spinal counterpart. Some reticular nuclei are referred to as vital centres since they are concerned with regulation of cardiac and respiratory activities; other parts of the reticular formation are essential for cerebral cortical arousal and the maintenance of consciousness, or are involved in the regulation of muscle tone, posture and reflex activities. The brain stem is the site of termination of numerous ascending and descending fibres and is traversed by many others. The spinothalamic tract (spinal lemniscus), medial lemniscus (see Fig. 18.10) and the trigeminothalamic tracts (see Fig. 19.16) all ascend through the brain stem to reach the thalamus Prominent corticospinal projections descend through the brain stem (see Fig. 18.17) and corticobulbar projections end within it.
OVERVIEW OF CRANIAL NERVES AND CRANIAL NERVE NUCLEI
The cranial nerves are the routes by which the brain receives information directly from, and controls the functions of, structures which are located mainly, although not exclusively, within the head and neck. All but two of the twelve pairs of cranial nerves attach to the brain stem. Below is a brief overview of the cranial nerves (consult also Table 15.1), their associated cranial nerve nuclei, and some of the brain stem reflexes to which they contribute. The cranial nerves are described in detail on a regional basis in appropriate chapters.
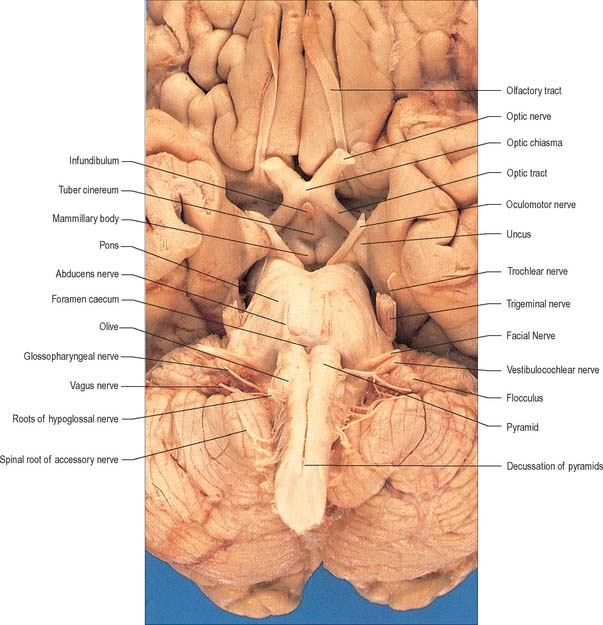
The cranial nerves are individually named and numbered (using Roman numerals) in a rostro-caudal sequence, reflecting their order of attachment to the brain. The first cranial nerve (olfactory) terminates directly in cortical and subcortical areas of the frontal and temporal lobes. It is closely associated functionally with the limbic system and is described in that context (Ch. 23). The fibres of the second cranial nerve (optic) pass into the optic chiasma where the centrally located fibres decussate; all of the fibres emerge as the optic tract, which terminates in the lateral geniculate nucleus of the thalamus. Cranial nerves III (oculomotor) and IV (trochlear) attach to the midbrain. Cranial nerve V (trigeminal) attaches to the pons, medial to the middle cerebellar peduncle. Cranial nerves VI (abducens), VII (facial) and VIII (vestibulocochlear) attach to the brain stem at, or close to, the junction of the pons with the medulla. Cranial nerves IX (glossopharyngeal), X (vagus), the cranial part of XI (accessory) and XII (hypoglossal) all attach to the medulla.
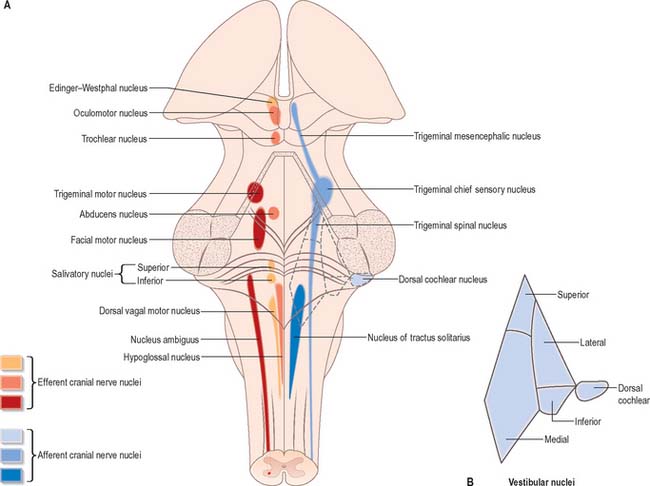
Cranial nerves III–XII, which attach to the brain stem, are associated with the brain stem cell groupings referred to collectively as the cranial nerve nuclei (Fig. 19.1). The nuclei are either the origin of efferent cranial nerve fibres or the site of termination of cranial nerve afferents. They are considered to be organized into six discontinuous longitudinal cell columns that correspond to columns that may be identified in the embryo (see Fig. 15.3). Three columns are ‘sensory’ and three are ‘motor’ in function.
MEDULLA OBLONGATA
EXTERNAL FEATURES AND RELATIONS
The medulla oblongata extends from just above the first pair of cervical spinal nerves to the lower border of the pons (Fig. 28.11). It is approximately 3 cm in length and 2 cm in diameter at its widest. The ventral surface of the medulla is separated from the basilar part of the occipital bone and apex of the dens by the meninges and occipito-axial ligaments. Caudally, the dorsal surface of the medulla occupies the midline notch between the cerebellar hemispheres.
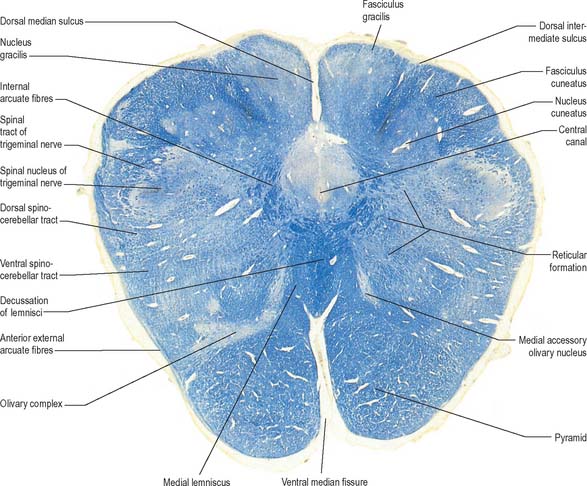
The ventral and dorsal surfaces of the medulla (Fig. 19.2, Fig. 19.3) possess a longitudinal median fissure and sulcus, respectively, which are continuous with their spinal counterparts. Caudally, the ventral median fissure is interrupted by the obliquely crossing fascicles of the pyramidal decussation. Rostrally, it ends at the pontine border in a diminutive depression, the foramen caecum. Immediately lateral to the ventral median fissure there is a prominent elongated ridge, the pyramid, which contains descending pyramidal, or corticospinal, axons. The lateral margin of the pyramid is indicated by a shallow ventrolateral sulcus. From this emerges, in line with the ventral spinal nerve roots, a linear series of rootlets which constitute the hypoglossal nerve. The abducens nerve emerges at the slightly narrowed rostral end of the pyramid, where it adjoins the pons. Caudally the pyramid tapers into the spinal ventral funiculus. Lateral to the pyramid and the ventrolateral sulcus there is an oval prominence, the olive (Fig. 19.2), which contains the inferior olivary nucleus. Lateral to the olive is the posterolateral sulcus. The glossopharyngeal, vagus and accessory nerves join the brain stem along the line of this sulcus, in line with the dorsal spinal nerve roots.
The spinal central canal extends into the caudal half of the medulla, migrating progressively more dorsally until it opens out into the lumen of the fourth ventricle. This divides the medulla into a closed part, which contains the central canal, and an open part, which contains the caudal half of the fourth ventricle (Fig. 19.3).
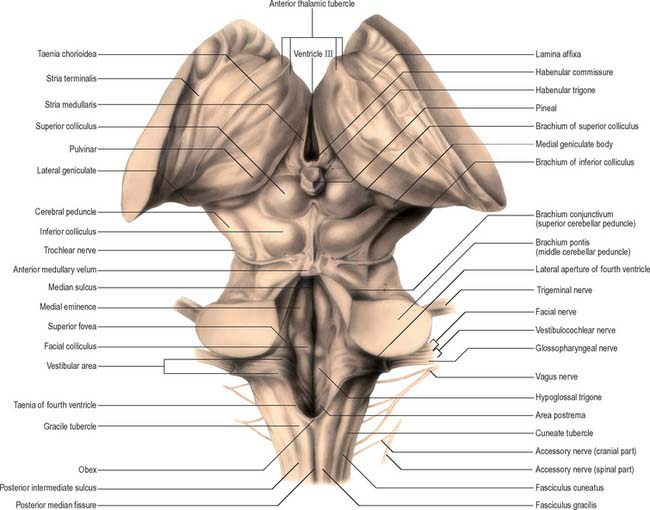
In the closed part of the medulla, a shallow dorsal intermediate sulcus, on either side of the dorsal median sulcus and continuous with its cervical spinal counterpart, indicates the location of the ascending dorsal columns (fasciculus gracilis and fasciculus cuneatus). The ascending fasciculi are at first parallel to each other, but at the caudal end of the fourth ventricle they diverge, and each develops an elongated swelling, the gracile and cuneate tubercles, produced by the subjacent nuclei gracilis and cuneatus respectively (Fig. 19.3, Fig. 19.4, Fig. 19.5). Most fibres in the fasciculi synapse with neurones in their respective nuclei, and these project to the contralateral thalamus, which, in turn, projects to the primary somaesthetic cortex (see Fig. 18.10). The inferior cerebellar peduncle forms a rounded ridge between the caudal part of the fourth ventricle and the glossopharyngeal and vagal rootlets. The peduncles of the two sides diverge and incline to enter the cerebellar hemispheres, where they are crossed by the striae medullares which run to the dorsal median sulcus of the ventricular floor (Fig. 19.3). Here also the peduncles form the anterior and rostral boundaries of the lateral recess of the fourth ventricle. This becomes continuous with the subarachnoid space through the lateral apertures of the fourth ventricle, or foramina of Luschka. A tuft of choroid plexus, continuous with that of the fourth ventricle, protrudes from the foramina on either side.
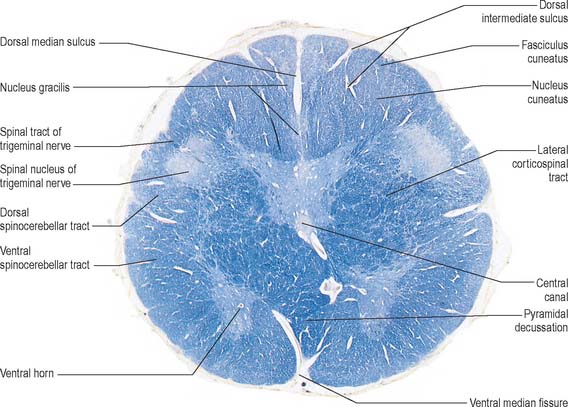
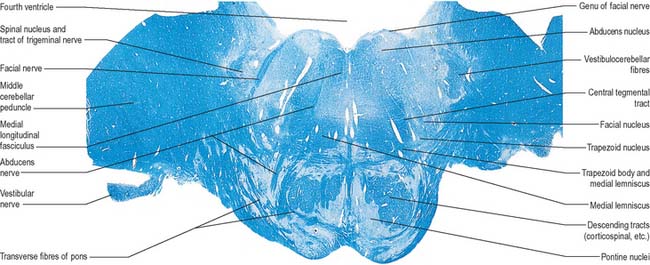
Fig. 19.4 Transverse section through the medulla oblongata at the level of the pyramidal decussation. Figs 19.4–19.6, 19.9–19.11, 19.18 and 19.19 are all prepared using a stain for myelinated nerve fibres (blue).
INTERNAL STRUCTURE
Transverse section of the medulla at the level of the pyramidal decussation
A transverse section across the lower medulla oblongata (Fig. 19.4) intersects the dorsal, lateral and ventral funiculi, which are continuous with their counterparts in the spinal cord. The ventral funiculi are separated from the central grey matter by corticospinal fibres, which cross in the pyramidal decussation to reach the contralateral lateral funiculi (see Fig. 19.8). The decussation displaces the central grey matter and central canal dorsally. Continuity between the ventral grey column and central grey matter, which is maintained throughout the spinal cord, is lost. The column subdivides into the supraspinal nucleus (continuous above with that of the hypoglossal nerve), which is the efferent source of the first cervical nerve, and the spinal nucleus of the accessory nerve, which provides some spinal accessory fibres and merges rostrally with the nucleus ambiguus.
Transverse section of the medulla at the level of the decussation of the medial lemniscus
The medullary white matter is rearranged above the level of the pyramidal decussation (Fig. 19.5). The pyramids form two large ventral bundles flanking the ventral median fissure on the ventral surface of the medulla.
They contain corticospinal fibres of ipsilateral origin. The nucleus gracilis is prominent on the dorsal aspect, with diminishing numbers of fibres of the fasciculus gracilis located on its dorsal, medial and lateral margins. The nucleus cuneatus is well developed. Both nuclei retain continuity with the central grey matter at this level, but this is lost more rostrally. First-order afferent fibres contained within the fasciculi gracilis and cuneatus synapse upon neurones in their respective nuclei. Second-order axons emerge from the nuclei as internal arcuate fibres, at first curving ventrolaterally around the central grey matter and then ventromedially between the spinal tract of the trigeminal nerve and the central grey matter. The fibres decussate in the midline thereafter forming the medial lemniscus which ascends to the thalamus. The decussation of internal arcuate fibres is located dorsal to the pyramids and ventral to the central grey matter, which is therefore more dorsally displaced than in the previous section.
Transverse section of the medulla at the caudal end of the fourth ventricle
A transverse section at the lower end of the fourth ventricle (Fig. 19.6) shows some new features together with most of those already described. The total area of grey matter is increased by the presence of the large olivary nuclear complex and nuclei of the vestibulocochlear, glossopharyngeal, vagus and accessory nerves.
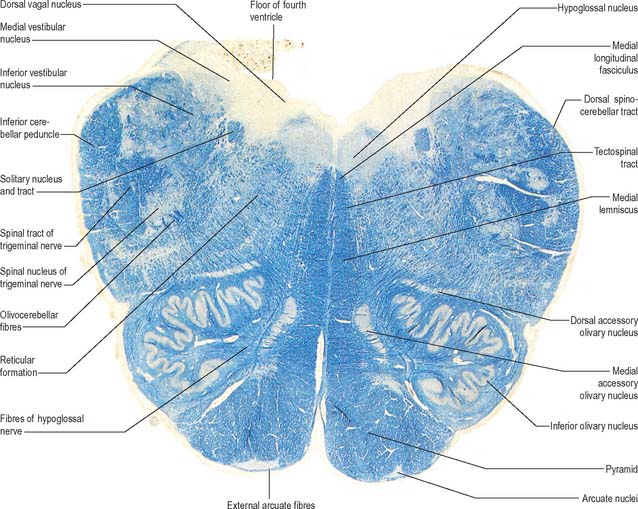
Fig. 19.6 Transverse section through the medulla oblongata at the caudal end of the fourth ventricle.
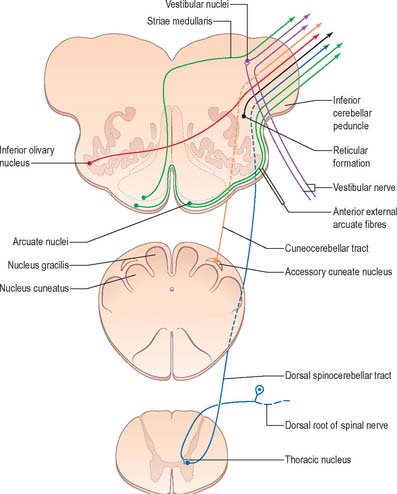
A smooth, oval elevation, the olive, lies between the ventrolateral and dorsolateral sulci of the medulla. It is formed by the underlying inferior olivary complex of nuclei, and lies lateral to the pyramid, separated from it by the ventrolateral sulcus and emerging hypoglossal nerve fibres. The roots of the facial nerve emerge between its rostral end and the lower pontine border, in the cerebellopontine angle. The arcuate nuclei are curved, interrupted bands, ventral to the pyramids, and are said to be displaced pontine nuclei. Anterior external arcuate fibres and those of the striae medullares are derived from them. They project mainly to the contralateral cerebellum through the inferior cerebellar peduncle (Fig. 19.7).
The inferior olivary nucleus is an irregularly crenated mass of grey matter with a medially directed hilum, through which numerous fibres enter and leave the nucleus. It has prominent connections with the cerebellum and is described more fully in Chapter 20.
The spinocerebellar, spinotectal, vestibulospinal, rubrospinal and lateral spinothalamic (spinal lemniscal) tracts all lie in the ventrolateral area of the medulla at this level. The tracts are limited dorsally by the nucleus of the spinal tract of the trigeminal and ventrally by the pyramid.
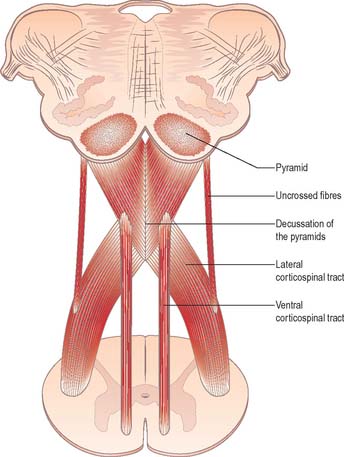
Pyramidal tract
Each pyramid contains descending corticospinal fibres, derived from the ipsilateral cerebral cortex, which have traversed the internal capsule, midbrain and pons (Fig. 19.8). Approximately 70–90% of the axons leave the pyramids in successive bundles, crossing in and deep to the ventral median fissure as the pyramidal decussation. In the rostral medulla fibres cross by inclining ventromedially, whereas more caudally they pass dorsally, decussating ventral to the central grey matter. The decussation is orderly, such that fibres destined to end in the cervical segments cross first. Fibres continue to pass dorsally as they descend, and reach the contralateral spinal lateral funiculus as the crossed lateral corticospinal tract. Most uncrossed corticospinal fibres descend ventromedially in the ipsilateral ventral funiculus, as the ventral corticospinal tract. A minority run dorsolaterally to join the lateral corticospinal tracts as a small uncrossed component. The corticospinal tracts display somatotopy at almost all levels. In the pyramids the arrangement is like that at higher levels, in that the most lateral fibres subserve the most medial arm and neck movements. Similar somatotopy is ascribed to the lateral corticospinal tracts within the spinal cord.
Dorsal column nuclei
The accessory cuneate nucleus, dorsolateral to the cuneate nucleus, is part of the spinocerebellar system of precerebellar nuclei (Fig. 19.7). It contains large neurones like those in the spinal thoracic nucleus and receives the lateral fibres of the fasciculus cuneatus, which carry proprioceptive impulses from the upper limb that enter the cervical spinal cord rostral to the thoracic nucleus. The accessory cuneate neurones give rise to the posterior external arcuate fibres that enter the cerebellum by the ipsilateral inferior cerebellar peduncle in the cuneocerebellar tract. A group of neurones, nucleus Z, identified in animals between the upper pole of the nucleus gracilis and the inferior vestibular nucleus, is said to be present in the human medulla. Its input is probably from the dorsal spinocerebellar tract, which carries proprioceptive information from the ipsilateral lower limb, and it projects through internal arcuate fibres to the contralateral medial lemniscus.
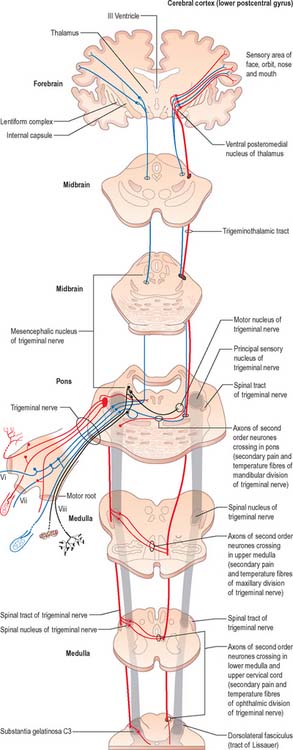
Trigeminal sensory nucleus
On entering the pons, the fibres of the sensory root of the trigeminal nerve run dorsomedially towards the principal sensory nucleus, which is situated at this level (Fig. 19.9). Before reaching the nucleus approximately 50% of the fibres divide into ascending and descending branches – the others ascend or descend without division. The descending fibres, of which 90% are less than 4 μm in diameter, form the spinal tract of the trigeminal nerve, which reaches the upper cervical spinal cord. The tract embraces the nucleus of the spinal tract of the trigeminal (spinal trigeminal nucleus; Fig. 19.4, Fig. 19.5, Fig. 19.6, Fig. 19.10, Fig. 19.11). There is a precise somatotopic organization in the tract. Fibres from the ophthalmic division of the trigeminal lie ventrolaterally, those from the mandibular division lie dorsomedially, and the maxillary fibres lie between them. The tract is completed on its dorsal rim by fibres from the sensory roots of the facial, glossopharyngeal and vagus nerves. All of these fibres synapse in the nucleus caudalis.
Fibres of the glossopharyngeal, vagus and facial nerves subserving common sensation (general visceral afferent) form a column dorsally within the spinal tract of the trigeminal nerve and synapse with cells in the lowest part of the spinal trigeminal nucleus. Consequently, operative section of the dorsal part of the spinal tract results in analgesia that extends to the mucosa of the tonsillar sinus, the posterior third of the tongue and adjoining parts of the pharyngeal wall (glossopharyngeal nerve), and the cutaneous area supplied by the auricular branch of the vagus.
Vagal nucleus
The vagal nucleus (also known as the dorsal motor nucleus of the vagus) lies dorsolateral to the hypoglossal nucleus, from which it is separated by the nucleus intercalatus. It extends caudally to the first cervical spinal segment and rostrally to the open part of the medulla under the vagal triangle of the floor of the fourth ventricle (Fig. 19.6).
Hypoglossal nucleus
The prominent hypoglossal nucleus lies near the midline in the dorsal medullary grey matter. It is approximately 2 cm long. Its rostral part lies beneath the hypoglossal triangle in the floor of the fourth ventricle (Fig. 19.3) and its caudal part extends into the closed part of the medulla.
Several smaller groups of cells lie near the hypoglossal nucleus (perihypoglossal nuclei), but none is known for certainty to be connected with the hypoglossal nerve or nucleus. They include the nucleus intercalatus, sublingual nucleus, nucleus prepositus hypoglossi and nucleus paramedianus dorsalis (reticularis). Gustatory and visceral connections are attributed to the nucleus intercalatus.
Inferior olivary nucleus
The olivary nuclear complex consists of the large inferior olivary nucleus and the much smaller medial accessory and dorsal accessory olivary nuclei (Fig. 19.6). They are the so-called precerebellar nuclei, a group that also includes the pontine, arcuate, vestibular, reticulocerebellar and spinocerebellar nuclei, all of which receive afferents from specific sources and project to the cerebellum. The inferior olivary nucleus contains small neurones, most of which form the olivocerebellar tract, which emerges either from the hilum or through the adjacent grey matter, to run medially and intersect the medial lemniscus (Fig. 19.6). Its fibres cross the midline, and sweep either dorsal to, or through, the opposite olivary nucleus. They intersect the lateral spinothalamic and rubrospinal tracts and the spinal trigeminal nucleus, and enter the contralateral inferior cerebellar peduncle, where they constitute its major component. Fibres from the contralateral inferior olivary complex terminate on Purkinje cells in the cerebellum as climbing fibres – there is a one-to-one relationship between Purkinje cells and neurones in the complex. Afferent connections to the inferior olivary nucleus are both ascending and descending. Ascending fibres, mainly crossed, arrive from all spinal levels in the spino-olivary tracts and via the dorsal columns. Descending ipsilateral fibres come from the cerebral cortex, thalamus, red nucleus and central grey of the midbrain. In part the two latter projections make up the central tegmental tract (fasciculus).
The medial accessory olivary nucleus is a curved grey lamina, concave laterally, between the medial lemniscus and pyramid and the ventromedial aspect of the inferior olivary nucleus. The dorsal accessory olivary nucleus is a similar lamina, dorsomedial to the inferior olivary nucleus. Both nuclei are connected to the cerebellum. The accessory olivary nuclei are phylogenetically older than the inferior olivary nucleus, and they are connected with the paleocerebellum. In all connections, cerebral, spinal and cerebellar, the olivary nuclei display specific topographical organization (Ch. 20).
Nucleus solitarius
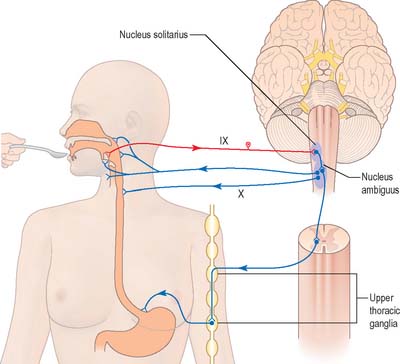
Swallowing and gag reflexes
During the normal processes of eating and drinking, passage of material to the rear of the mouth stimulates branches of the glossopharyngeal nerve in the oropharynx (Fig. 19.12). This information is relayed via the nucleus solitarius to the nucleus ambiguus, which contains the motor neurones innervating the muscles of the palate, pharynx, and larynx. The nasopharynx is closed off from the oropharynx by elevation of the soft palate. The larynx is raised, its entrance narrowed and the glottis is closed. Peristaltic activity down the oesophagus to the stomach is mediated through the pharyngeal plexus.
Nucleus ambiguus
The nucleus ambiguus is a group of large motor neurones, situated deep in the medullary reticular formation (see Fig. 19.10). It extends rostrally as far as the upper end of the vagal nucleus while caudally it is continuous with the nucleus of the spinal accessory nerve. Fibres emerging from it pass dorsomedially, then curve laterally. Rostral fibres join the glossopharyngeal nerve. Caudal fibres join the vagus and cranial accessory nerves and are distributed to the pharyngeal constrictors, intrinsic laryngeal muscles and striated muscles of the palate and upper oesophagus.
The nucleus ambiguus contains several cellular subgroups, and some topographical representation of the muscles innervated has been established. Individual laryngeal muscles are innervated by relatively discrete groups of cells in more caudal zones. Neurones that innervate the pharynx lie in the intermediate area, and neurones that innervate the oesophagus and soft palate are rostral.