Arthritis-Related Cysts (Osteoarthrotic Cysts, Subchondral Cysts of Degenerative Joint Disease and Rheumatoid Arthritis); Geodes
Cysts associated with degenerative joint disease or rheumatoid arthritis may result from subchondral remodeling bone or from synovial pathology extending into bone. Subchondral cysts in degenerative joint disease are usually multiple, vary in size and shape, and may be quite large (
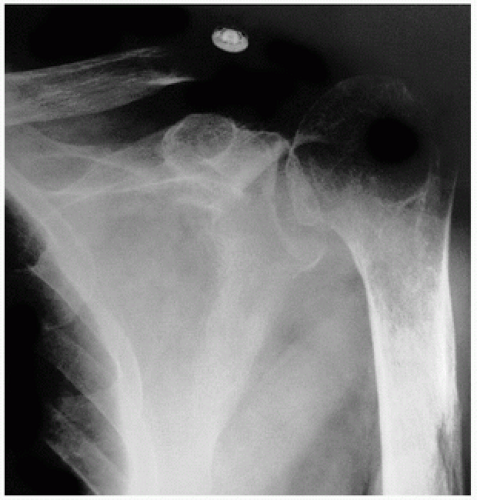
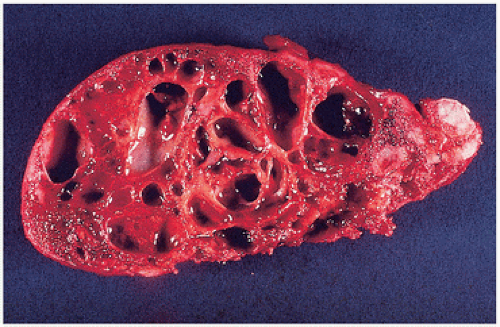
Fig. 6.1). The term geode has been used by radiologists for the spherical cysts adjacent to articular surfaces in arthritic joints (
2). Osteoarthrotic cysts occur in bone on both sides of the joint, are usually centrally located, and often occur in weight-bearing bones. They are usually pyriform in shape and multilocular. Although they may be contiguous with the joint, usually a fine, thin subchondral bone plate separates the joint space. The end of the bone shows the typical changes of degenerative joint disease, including eburnation (denuded articular cartilage and thickened polished subchondral bone surfaces), joint space narrowing, and osteophyte formation (
Fig. 6.2).
Large subchondral bone cysts may mimic tumors roentgenographically (
3). Despite the fact that subchondral cysts are a common finding in osteoarthritis, their etiology remains obscure.
Two etiologic theories are invoked to explain the origin of bone cysts: one of a synovial origin, supported by the similarities of cyst fluid to synovial fluid and the presence of abnormal articular cartilage near cysts, and a second theory suggesting cyst origin in the abnormal remodeling and loss of subchondral bone that occurs as part of the osteoarthritic process. It is likely that osteoarthritic cysts are the result of stress-induced resorption (
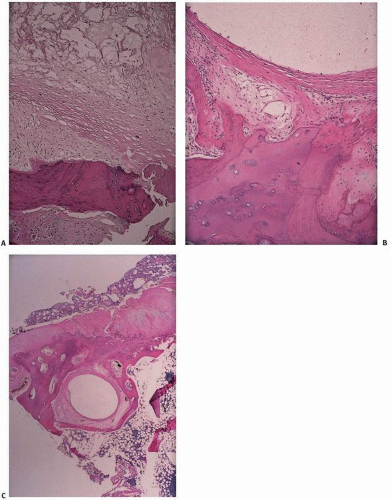
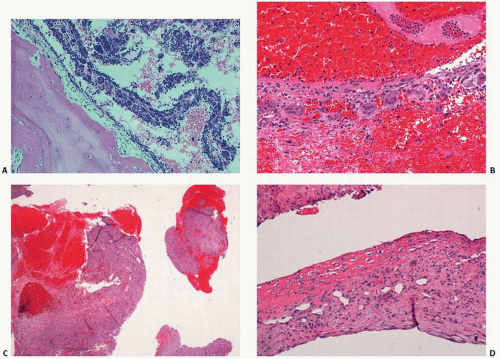
4). Microscopically, cysts vary from myxomatous changes of the underlying marrow with extensive bone remodeling to walled-off, fibrous, membranous cavities with or without extensions to the surface (
Fig. 6.3).
Eggers cysts are acetabular cysts associated with degenerative joint disease of the hip (
5). Osteoarthritis-related cysts at other joints are also well described (
6). The dorsal cysts of the distal interphalangeal joints seen in middle-aged women and associated with joint space narrowing and Heberden nodes (osteophytes) are thought to arise from degeneration of the capsular tissue of the joint that spreads subsequently to bone; like most cysts associated with degenerative joint disease, they are thin-walled and contain a clear, viscid fluid. Myxomatous degenerating fibrous tissue identical to a ganglion is the rule.
Cysts may also occur in patients with rheumatoid arthritis and reach considerable size (
2). Soft tissue cysts, including popliteal and synovial cysts, are a hallmark of rheumatoid arthritis (
7). Cysts are also associated with hemophilia, osteonecrosis, and pigmented villonodular synovitis and are also characteristic of these lesions.
Stark et al. (
8) have called attention to the features that differentiate osteoarthritic cysts from intraosseous ganglia (
Table 6.2). An increased localized signal on magnetic resonance imaging (MRI) reflects the fluid content of these lesions (
9).
Cysts have also been described in miscellaneous conditions, including iatrogenic injury following contrast studies, so-called pneumocysts (
10).
Ganglionic Cystic Defects of Bone
Intraosseous ganglionic cysts are solitary, uniloculated or multiloculated, lytic defects (
20,
21). They are well demarcated with a sclerotic rim at the epiphyseal ends of long bones, commonly the
medial malleolus of the ankle, the femoral head, proximal tibia, carpal bones, and distal end of the ulna. Despite its proximity to a joint, the lesion rarely communicates with the joint. Two types have been described, one of which is purely intraosseous. Occasionally, an overlying soft tissue ganglion is present, which may communicate with the intraosseous ganglion; such lesions constitute the second group. First named by Crabbe, ganglionic bone cysts can be distinguished from the subchondral bone cysts seen in association with degenerative joint disease because the adjacent joint is essentially unremarkable (
8) (
Table 6.2). The relative large size, location in a non-weight-bearing region of a joint, and lack of communication with the adjacent joint often distinguish ganglionic bone cysts from those of degenerative joint disease. Patients are middle-aged (mean age, 44) and usually present with mild, intermittent, localized pain made worse by weight bearing. Swelling may be present or a slowly growing mass noted. Nearly half of patients are asymptomatic.
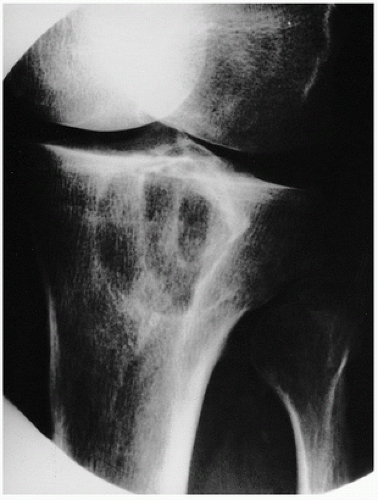
Roentgenographically, there is a well-circumscribed radiolucent lesion in the subchondral epiphyseal region of bone, which may extend into the metaphysis (
Fig. 6.6).
MRI of soft tissue ganglia has revealed the following:
T1, hypodense compared with muscle and fat
T2, increased relative to T1, similar to fat
MRI of ganglionic bone cyst has revealed the following (
22):
T1, low signal intensity
T2, high signal intensity
Grossly, the lesion is a unilocular or multilocular cyst lined by thick, fibrous membrane and filled with a clear or yellowish gelatinous or mucinous material (
Fig. 6.7). The surrounding bone shows sclerosis.
Microscopically, the wall is composed of a dense, fibrous connective tissue layer with focal mucoid degeneration, myxoid connective tissue, and flattened membranes with sparse and inconspicuous lining cells (
Fig. 6.7). Occasional mononuclear inflammatory cells may be noted. Adjacent bone shows thickening and active remodeling.
Ganglionic bone cysts are effectively treated by curettage. The recurrence rate is low.
Schajowicz proposed that these cysts, not unlike their soft tissue counterparts, occur when repeated trauma leads to aseptic necrosis and mucoid degeneration. Others, recognizing the predilection of soft tissue ganglia for sites adjacent to or contiguous with tendons, ligaments, or tenosynovial structures, have suggested herniation or entrapment of soft tissue in tight anatomic compartments as the initial event. Entrapped tissue undergoes vascular compromise and other metabolic degeneration. The ensuing cellular reaction involves mesenchymal cells capable of producing mucinous material. Ganglia take shape and act subsequently as a mass-occupying lesion, eroding adjacent cortical bone (
22). This theory would explain the predilection for the epiphyseal and metaphyseal sites
(where tendons insert) in bone. Remodeling cortical bone may subsequently seal itself off. However, an intraosseous etiology, in which lesions arise de novo, remains plausible.
Miscellaneous Cysts
When one considers the myriad associations of cystic development in bone, a reasonable conclusion is that it is a common bone remodeling event. Cysts can complicate a wide range of tumors, degenerative joint changes (osteoarthritis, tarsal coalition), and bleeding diatheses.
The term
intraosseous pneumatocyst has been used to describe a localized collection of gas, usually adjacent to a sacroiliac joint, with no significant bone destruction or soft tissue mass (
23).
Gas-filled intradural cysts have been described and, although the intraspinal gas may disappear spontaneously, unremitting radicular pain and neurologic deficits may warrant surgical intervention (
24).
Gas observed on imaging has been referred to as “vacuum phenomenon.” In the spine, this is thought to be due to the creation of a negative pressure effect when there is distraction of the disc space. Gas (primarily nitrogen) is thus drawn into the disc from surrounding tissues.
Tarlov cysts are perineural cysts due to cystic dilatation of the perineurium and endoneurium of nerve roots filled with cerebrospinal fluid (
25).
The subpubic cartilaginous cysts are cystic lesions arising secondary to degenerative changes in the symphysis pubis, and are most commonly seen in multiparous women (
26).
The fact that cysts have been described at so many sites of musculoskeletal insertions, and at sites such as femoroacetabular impingement regions, and rotator cuff tears suggests that there are
protean causes of tissue trauma, irritation, and degeneration that can lead to cystic pathology in both bone and soft tissue.
Unicameral Bone Cysts (Solitary Cysts, Simple Bone Cysts)
A simple bone cyst is a benign, solitary, cystic defect in the metaphyseal region of long bones in children and adolescents (
27). The most common location coming to clinical attention is the proximal humerus and, less often, the proximal femur (
Fig. 6.8). The presence of these cysts in other bones, such as the pelvis and calcaneus, is well described (
28).
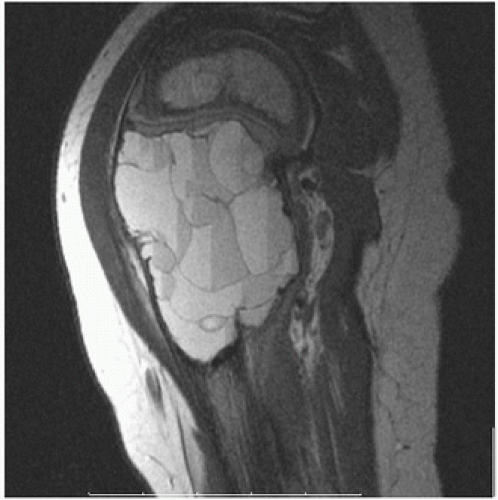
Roentgenographically, the lesion classically presents as a solitary metaphyseal radiolucency abutting on but not involving the growth plate (
Fig. 6.9). A pseudoloculated appearance resulting from irregular remodeling of the residual host trabecular bone and thinning of the cortex is often seen. The remodeling effect on cortical bone with attenuation may result in fracture and thus pain, the most common cause of clinical presentation. Most simple bone cysts are asymptomatic and go undetected.
UBCs are usually found in children ages 4 to 10 years, but at times can be detected in older persons, up to age 40 years. Unless complicated by a fracture, these cysts tend to be asymptomatic, even though at times a dull ache is described, especially by the older patients.
These cysts are most common in the proximal metaphyses of the humerus, femur, and tibia. Other locations include the pelvis (usually the iliac wing), calcaneus, and long bones of the forearm. The lesion appears as a lucency adjacent to the growth plate. The simple cyst is considered active as long as it touches the growth plate. The formation of new bone at the growth plate causes an apparent displacement of the cyst toward the diaphysis. The extent of displacement is thought to be a function of the growth potential of the physis nearby. Therefore, whereas a benign bone cyst of the proximal humerus can be displaced a considerable distance into the shaft of the bone, a benign bone cyst of the proximal femur is displaced a small distance into the shaft, as most of the longitudinal growth of the humerus occurs at its proximal end, and of the femur at its distal end. The cyst is regarded as latent once it is separated from the growth plate by at least 1 to 2 cm. Simple bone cysts do not typically extend into the epiphyses but can certainly do so (
29).
Simple bone cysts are seen roentgenographically as well-defined lucencies, with or without minimal surrounding sclerosis. The lucency is homogeneous, without calcifications or septations. There is usually mild expansion of the bone. The lesion is centrally located in the shaft of a tubular bone, so that in cross-sectional imaging on computed tomography (CT) or MRI, the cyst is equidistant from all cortices. Fractures are common in simple bone cysts, and small bone fragments can be displaced into the cyst in such situations. Although the risk of a pathologic fracture in a UBC is hard to define, in the calcaneus it has been estimated that a calcaneal cyst covering 100 percent of the cross section in a coronary plane and at least 30 percent in a sagittal plane is at significant risk for fracture (
30).
In a study of predominantly humeral bone cysts, Mary et al. (
31) estimated an 80- to 100-percent risk of fracture if the cyst had the following characteristics:
Approximately 70 percent of UBCs present initially with a pathologic fracture. Before the fracture, they are usually asymptomatic. In most cases, the fracture will heal, and the lesion can be addressed at the time of fracture or after the fracture is healed. The fracture heals in about 6 weeks, but the cyst often persists. Only about 10 percent heal completely after a fracture (
32).
Specific treatment of the cyst is usually delayed until the fracture is well healed. Closed reduction and immobilization for 4 to 6 weeks usually suffices, but instability and displaced fractures of the femur may require internal fixation.
There may be a direct relationship between cyst volume and ability to heal, with less healing potential in large cysts (
33). CT demonstrates the benign bone cyst as a lucent, centrally located lesion, with thinning and mild to moderate expansions of the adjacent cortex. Measurements of the CT density of the lesion (e.g., Hounsfield units) yield a value in the low positive range, typical for fluid (
34). MRI demonstrates a centrally located lesion, with low signal intensity in the T1-weighted images and very high signal intensity in the T2-weighted images, typical for fluid (
Fig. 6.10). The presence of a fracture and hemorrhage can result in a mixture of blood and fluid in the cyst, increasing its density in the CT measurements and also changing the signal intensity on MRI. Radionuclide bone scan tends to show mildly increased uptake in the periphery of an uncomplicated bone cyst and significantly increased uptake if a fracture is present. Fractures can cause periosteal reaction at the edges of the cyst. The expanding cyst is also often accompanied by periosteal reaction at the site of junction of the expanded cortex with the cortex of the uninvolved portion of the bone.
*The Kaelin index is a measurement using software to assess the cyst surface area as a ratio of shaft diameter (S/d2).
The fallen fragment or “fallen leaf” sign, first described by Reynolds in 1969, may be useful in identifying the lesion as a cyst, and can be seen in 20 percent of cases (
35). It refers to the gravitational settling of a small fragment of bone within the dependent portion of a lytic lesion, implying that the lesion is hollow. By using several roentgenograms of different postures of the involved extremity, intracavitary dislodged fragments of trabecular bone may be shown to move freely within the cavity (
Fig. 6.11).
A “rising bubble” sign has also been described as a corollary to the fallen fragment sign, and it indicates the presence of a gas bubble ascending to the most nondependent margin of a fractured lytic lesion, implying that the lesion is hollow and devoid of solid internal matrix or tissue (
36). And so, in UBCs, the fragment falls as the bubble rises.
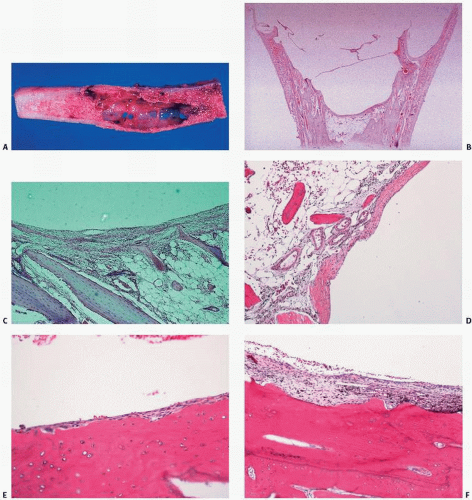
UBCs can have a bluish appearance when exposed operatively. Grossly, an unaltered lesion consists of a cyst filled with clear or straw-colored fluid and lined with a thin, fibrous membrane (
Fig. 6.12). The fibrous membrane that lines a simple bone cyst is typically sparsely cellular. However, because fracture is a common
complication, one may observe “secondary” changes, such as hemorrhage and hemosiderin deposition, granulation tissue, cholesterol clefts, fibrin, calcification, and reactive bone. In such instances, the lesion may mimic histologically an ABC or even a giant-cell tumor (GCT). Other possible histologic features include a rarely observed cementoma-like change. The cementum material in cysts has been studied and is thought to represent a peculiar acellular or hypocellular type of bone (
37,
38). Lesions composed purely of cementum material, “cementomas,” are well described in long bones (
39).
Cyst contents have been studied. Gerasimov et al. (
40) showed cyst fluid to contain higher levels of lysosomal enzymes than serum, and so postulated an enzymatic role in bone cyst growth (
Fig. 6.13). Komiya et al. (
41,
42) measured bone resorptive factors in cyst fluid, including prostaglandins, interleukin 1β, levels of nitrate and nitrite, and proteolytic enzymes. The cyst membrane has been shown to stain for tumor necrosis factor α, and interleukins 1β and 6. Other factors that most likely affect cyst growth are increases in colloidal osmotic pressure of the cyst cavity and disturbances in microcirculation.
Although Cohen (
43) has postulated blockage in the drainage of interstitial fluid in a rapidly growing and rapidly remodeling portion of cancellous bone, Chiriga et al. (
44) found higher internal pressure within the involved bone postulating venous obstruction.
Recurrence after en bloc resection would be exceptional, but has raised the possibility of the role of surface periosteal tissue (
45).
Although benign, these cysts recur at a high rate after surgery, particularly in children younger than 10 years, in whom the lesion is characteristically juxtaepiphyseal in location. Pathologic fracture may heal without surgical intervention and may even lead to spontaneous resolution.
Unlike UBCs, where pathologic fracture is a relatively common presenting problem, pathologic fracture in ABCs is an infrequent event, occurring in approximately 10 percent of patients.
The natural history of UBC is highly variable. Originating in the metaphysis, it may be observed developing progressively down the shaft of the proximal and distal ends of the bone as the host bone grows away during endochondral ossification (
Fig. 6.14). Normal bone formation by the adjoining physis can be an indicator of successful resolution of a cyst.
Although actual penetration of the epiphyseal plate with the extension of a UBC into the epiphysis is infrequent (2 percent), limb growth can be affected in limb shortening more likely in the humerus than in the femur.
UBCs tend to resolve as maturity approaches, and although they can be found in young adults, they are usually asymptomatic and not active (
45).
Healing has been accompanied by roentgenographically detectable filling of the defect by bone or even fat (
46).
Treatment strategies for UBC include a broad range of approaches and techniques and can be generally classified into:
intralesional injections,
curettage procedures with or without adjuvants,
procedures to disrupt the cyst wall lining,
decompression procedures with Kirschner wires or cannulated screws,
procedures to provide structural stabilization (e.g., flexible intramedullary nailing),
combination strategies involving the above.
Recurrence rates following various procedures have been tabulated for ABCs (
47) (
Table 6.3). Steroid injections may stabilize the lysosomal membranes that have been identified in some studies (
40,
48). Other substances have also been injected into UBCs including:
Ethibloc is a thrombogenic and fibrogenic agent consisting of an alcohol solution of zein, a corn protein (
54). Injected cement has been commonly used, but concern that chondrolysis may occur has been an issue (
55).
The plethora of injected materials used to treat UBCs suggests a lack of consensus on proven efficacies.
To counteract reputed increases in intracavitary pressure, decompression by drilling multiple cortical burr holes has been used (
56). Effects of decompression on cavity metabolism, microcirculation, and periosteal stimulation of new bone remodeling have all been postulated.
Zoledronate, a bisphosphonate, has been used to induce apoptosis of the new osteoclast cells that may inhabit the cyst membrane (
57).
Bone grafts after curettage have been a standard approach to the treatment of large lesions, particularly in weight-bearing bones. Bone graft augmenters, such as high-porosity hydroxyapatite, have also been used (
58).
Strategies that combine multiple approaches to treatment testify to the ongoing attempt to find the optimum treatment. Hou et al. (
59) found the highest rate or radiographically determined healing in those treated with a minimally invasive curettage, ethanol cauterization, disruption of the cystic boundary, insertion of a synthetic calcium sulfate bone graft substitute, and placement of a cannulated screw to provide drainage.
Endoscopic curettage as opposed to open surgical treatment is being explored (
60).








 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access