Bone and Joint Infections
KEY CONCEPTS
![]() The most common cause of osteomyelitis (particularly that acquired by hematogenous spread) and infectious arthritis is Staphylococcus aureus.
The most common cause of osteomyelitis (particularly that acquired by hematogenous spread) and infectious arthritis is Staphylococcus aureus.
![]() Culture and susceptibility information are essential as a guide for antimicrobial treatment of osteomyelitis and infectious arthritis.
Culture and susceptibility information are essential as a guide for antimicrobial treatment of osteomyelitis and infectious arthritis.
![]() Joint aspiration and examination of synovial fluid are extremely important to evaluate the possibility of infectious arthritis.
Joint aspiration and examination of synovial fluid are extremely important to evaluate the possibility of infectious arthritis.
![]() The most important treatment modality of acute osteomyelitis is the administration of appropriate antibiotics in adequate doses for a sufficient length of time.
The most important treatment modality of acute osteomyelitis is the administration of appropriate antibiotics in adequate doses for a sufficient length of time.
![]() Antibiotics generally are given in high doses so that adequate antimicrobial concentrations are reached within infected bone and joints.
Antibiotics generally are given in high doses so that adequate antimicrobial concentrations are reached within infected bone and joints.
![]() The standard duration of antimicrobial treatment for acute osteomyelitis is 4 to 6 weeks.
The standard duration of antimicrobial treatment for acute osteomyelitis is 4 to 6 weeks.
![]() Oral antimicrobial therapies can be used for osteomyelitis to complete a parenteral regimen in children who have had a good clinical response to IV antibiotics and in adults without diabetes mellitus or peripheral vascular disease when the organism is susceptible to the oral antimicrobial, a suitable oral agent is available, and compliance is ensured.
Oral antimicrobial therapies can be used for osteomyelitis to complete a parenteral regimen in children who have had a good clinical response to IV antibiotics and in adults without diabetes mellitus or peripheral vascular disease when the organism is susceptible to the oral antimicrobial, a suitable oral agent is available, and compliance is ensured.
![]() The three most important therapeutic approaches to the management of infectious arthritis are appropriate antibiotics, joint drainage, and joint rest.
The three most important therapeutic approaches to the management of infectious arthritis are appropriate antibiotics, joint drainage, and joint rest.
![]() Monitoring of antibiotic therapy is important and typically involves noting clinical signs of inflammation, periodic white blood cell (WBC) counts, C-reactive protein, erythrocyte sedimentation rate (ESR) determinations, and radiographic findings.
Monitoring of antibiotic therapy is important and typically involves noting clinical signs of inflammation, periodic white blood cell (WBC) counts, C-reactive protein, erythrocyte sedimentation rate (ESR) determinations, and radiographic findings.
Bone and joint infections are comprised of two disease processes known, respectively, as osteomyelitis and septic or infectious arthritis. They are unique and separate infectious entities with different signs and symptoms and infecting organisms. Despite advances in therapy, these infections continue to cause significant morbidity from residual damage and chronic or recurring infections. Emphasis on initiating antibiotic therapy as soon as possible is important in reducing long-term complications.
EPIDEMIOLOGY
Osteomyelitis generally is an uncommon disease. One classic publication reported that 247 patients had osteomyelitis in a prominent American teaching hospital during a 4-year period.1 Acute osteomyelitis has an estimated annual incidence of 0.4 per 1,000 children.2 In adults, osteomyelitis caused by contiguous spread, including postoperative, direct puncture, and that associated with adjacent soft tissue infections, comprises 47% of infections. Hematogenous osteomyelitis comprises 19% of infections, and osteomyelitis occurring in patients with significant peripheral vascular disease comprises 34% of infections. A review of osteomyelitis cases based on duration of disease shows that acute disease constitutes 56% of patients and that chronic osteomyelitis, defined as having a previous hospitalization for the same infection, constitutes 44% of patients. Another classification system has defined acute osteomyelitis as <2 weeks duration, subacute as 2 to 4 weeks duration, and chronic as >4 weeks duration.
Infectious or septic arthritis is an inflammatory reaction within the joint space. Distinct from osteomyelitis, septic arthritis is one of the most common causes of new cases of arthritis. The incidence of proven or likely septic arthritis is 4 to 10 cases per 100,000 patient-years per year.3 The incidence of septic arthritis increases to 70 cases per 100,000 patient-years among patients that have rheumatoid arthritis.4
ETIOLOGY
Osteomyelitis
The most common method of classifying osteomyelitis is based on the mode of acquisition of the bone infection. Disease that results from spread through the bloodstream is termed hematogenous osteomyelitis, while that reaching the bone from an adjoining soft tissue infection is termed contiguous osteomyelitis. Patients with peripheral vascular disease are at risk for the development of contiguous osteomyelitis, and they present unique management features. Osteomyelitis that results from direct inoculation, such as from trauma, puncture wounds, or surgery, generally is also classified as inoculation osteomyelitis.
Osteomyelitis also can be classified based on the duration of the disease. Acute osteomyelitis describes infections of recent onset, usually several days to 1 week, whereas chronic infections are those of a longer duration. Some authors describe chronic infections as those with symptoms for more than 1 month before therapy, whereas other authors define chronic infections as relapse of an initial infection. Hematogenous osteomyelitis almost always involves one bone whereas contiguous osteomyelitis can present in multiple bones, especially when vascular insufficiency is an underlying risk factor.
Infectious Arthritis
Infectious arthritis can occur from many different types of microorganisms.5 Most infecting organisms produce an infection in a single joint, termed monoarticular infections; however, infections also can involve two or more joints.6 As with osteomyelitis, joint infections also can be classified according to the mechanisms by which the infecting organism reaches the joint. Infectious arthritis can result by spread from an adjacent bone infection, direct contamination of the joint space, or hematogenous dissemination. Hematogenous spread of the disease comprises the majority of infections; spread from osteomyelitis and direct inoculation is much less frequent. Septic arthritis is most prevalent in children and the elderly. Approximately, one-third of people with septic arthritis are children younger than 2 years of age.7
PATHOPHYSIOLOGY
Hematogenous Osteomyelitis
Hematogenous osteomyelitis is typically a disease of the growing bone in children and most cases occur in patients younger than 16 years of age. Table 96–1 summarizes the primary characteristics of osteomyelitis. Less commonly, these infections occur in adults. Osteomyelitis of the vertebrae is also acquired hematogenously and occurs most frequently in patients older than 50 years of age.8
TABLE 96-1 Types of Osteomyelitis, Age Distribution, Common Sites, and Risk Factors
Unique features of the anatomy and physiology of long bones appear to predispose them to become infected.9 Their vascular structure appears to predispose them for hematogenous infections that begin within the metaphyses (Fig. 96–1). The nutrient arteries of the long bones divide within the medullary canal of the bone into small arterioles. These end in hairpin turns near the growth plate and flow into veins, of much wider diameter, that drain the medullary cavity.1 An infection in hematogenous disease is initiated within the bend of the arterioles where there is considerable slowing of blood flow passing through the hairpin capillary loops. This sludging of blood flow allows bacteria present within the bloodstream to settle and initiate an inflammatory response. They have access to the bone by gaps in the endothelial layer and the absence of a basement membrane. In addition to these structural features, there also appears to be less active phagocytosis within the metaphysis. After the bacteria settle in the bone, avascular necrosis can occur from occlusion of the nutrient vessels and release of bacterial enzymes.
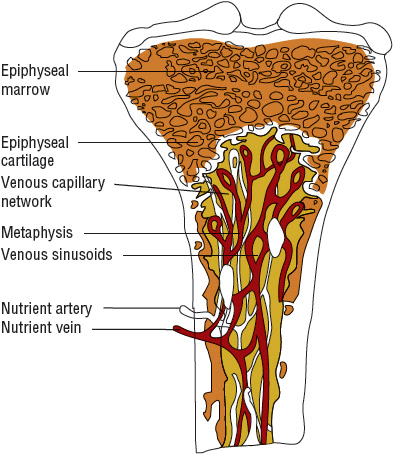
FIGURE 96-1 Cross section of normal bone.
In addition to these anatomic and functional features, there is some evidence that trauma is associated with developing an infection in specific bones. Children who develop hematogenous osteomyelitis may report some type of trauma before the onset of their symptoms and animal data indicate that traumatized bone is more likely to become infected than normal bone.
Once the infection is initiated, exudate begins to form within the bone marrow and the fluid accumulates under increased pressure. The age of the patient largely determines the next stage in the pathophysiology. In children older than 12 to 18 months, the infection that started in the metaphysis of a long bone is prevented from spreading into the epiphysis and the adjacent joint space because of the epiphyseal growth plate that acts as a physical barrier; however, the exudate often dissects from the medulla through the soft cortex to the subperiosteal space as the periosteum in these children is loosely attached to the underlying cortex. The periosteum is thick and not easily ruptured thus containing the pus in the subperiosteal space, sometimes forming a subperiosteal abscess. If there is significant damage to the periosteum, the pus can decompress into a soft-tissue abscess. The cortex obtains most of its blood supply from the periosteum and a subperiosteal abscess can impair the blood flow to the outer portion of the cortical bone resulting in a devitalized piece of dead bone termed a sequestrum. The elevated periosteum remains viable because its blood supply, derived from the overlying muscle, is unaffected. The raised periosteum will continue to produce bone; however, this new bone is now separated from the cortex because the periosteum has been raised from the infection. This new bone that is deposited under the periosteum is termed involucrum.
In adults, the periosteum is tightly bound to the cortex that is thick. These anatomic features generally cause the infections to remain intramedullary. As expected, subperiosteal abscess formations are less common in adults. The infection can spread to subperiosteal structures through the Haversian and Volkmann’s canals.
The vascular supply of long bones in neonates also has unique anatomic characteristics that affect their presentation. Bridging blood vessels go across the epiphyseal plate from the metaphysis into the epiphysis thus enabling an infection that started within the metaphyseal area to spread easily to involve the epiphyses and then into the joint.10 Therefore, in infants, not only can the infection spread to involve the periosteum and the shaft as in older children, but the infection also can spread directly to involve the joint.11
In children, hematogenous osteomyelitis typically involves a single bone and has a predilection for involvement of the long bones, such as the femur, tibia, humerus, and fibula.11 In contrast, neonatal infections commonly involve multiple bones. Vertebral infections are common in patients older than 50 years of age.12 Vertebral disease in young children usually involves the disk space and the two vertebral facets adjoining it because of the nature of the vascular supply of the vertebrae at that age. This entity is known as diskitis. Vertebral osteomyelitis involving the body of the vertebra can be seen in children older than 8 years of age.
Chronic osteomyelitis is more likely to occur if large segments of bone become avascular and necrotic. This results in a piece of devitalized bone to which antimicrobial delivery is impaired. As a result, this infection is prone to exacerbations and may lead to weakening of that bone or to the formation of draining sinuses to the skin.
![]() The bacteriology of hematogenous osteomyelitis is unique in that one pathogen, Staphylococcus aureus, is responsible for more than 80% of these infections, with group A Streptococci and Streptococcus pneumoniae accounting for a few cases. Kingella kingae, an organism that is part of the oral flora is emerging as a pathogen in children less than 3 years of age. Haemophilus influenzae type b (Hib), which used to be an important pathogen, has been almost completely eliminated with the use of the conjugate vaccine and is now a rare pathogen in bone and joint infections.9 Similarly, pneumococcal disease is anticipated to decrease in prevalence as invasive pneumococcal disease is prevented by the use of the conjugate pneumococcal vaccine in infants. While S. aureus is also the major pathogen in neonatal osteomyelitis, disease in this age group can also result from infections with group B streptococcus, and Escherichia coli. They are multifocal in half the cases.
The bacteriology of hematogenous osteomyelitis is unique in that one pathogen, Staphylococcus aureus, is responsible for more than 80% of these infections, with group A Streptococci and Streptococcus pneumoniae accounting for a few cases. Kingella kingae, an organism that is part of the oral flora is emerging as a pathogen in children less than 3 years of age. Haemophilus influenzae type b (Hib), which used to be an important pathogen, has been almost completely eliminated with the use of the conjugate vaccine and is now a rare pathogen in bone and joint infections.9 Similarly, pneumococcal disease is anticipated to decrease in prevalence as invasive pneumococcal disease is prevented by the use of the conjugate pneumococcal vaccine in infants. While S. aureus is also the major pathogen in neonatal osteomyelitis, disease in this age group can also result from infections with group B streptococcus, and Escherichia coli. They are multifocal in half the cases.
Vertebral osteomyelitis has several unique features and occurs most commonly in adults 50 to 60 years of age. The lumbar and thoracic regions are the locations of most infections. Hematogenous infections are most likely to develop in the vascular areas near the subchondral plate region of the vertebral body. Staphylococci cause approximately 60% of these infections; however, gram-negative organisms now play a significant role.13 These gram-negative organisms, particularly E. coli, most likely originate within the urinary tract. E. coli vertebral infections have been associated with urinary tract infections, positive urine cultures, and bacteremias. Mycobacterium tuberculosis and Coccidioides immitis/posadasii also are known to cause infections in the spine. Skin and respiratory tract infections are other sources of infection known to lead to vertebral infections.
While infections of the spine can involve the vertebrae in 1% to 2% of older children with osteomyelitis, they more commonly involve the disk space of the lumbar vertebrae in children less than 5 years of age.
Osteomyelitis in the IV drug user has unique features.14 More than 50% of such infections involve the vertebral column and less than 20% of infections are located in either the sternoarticular or pelvic girdle. Infections are much less frequent within the extremities. They also have an unusual spectrum of organisms with gram-negative organisms being responsible for 88% of infections. Pseudomonas aeruginosa, either singly or in combination with other organisms, is cultured in 78% of all infections. Klebsiella, Enterobacter, and Serratia species also can be found but less commonly. In addition, staphylococcal and streptococcal organisms are sometimes cultured.
Patients with sickle cell anemia and related hemoglobinopathies also represent a unique population in that two thirds of bone infections in these patients are caused by Salmonella species, while the rest are usually caused by staphylococci and other gram-negative organisms.15 Bowel infarctions from the sickle cell disease can facilitate the entry of salmonellae from the colon into the bloodstream with resultant hematogenous spread to the bone. Osteomyelitis in patients with sickle cell disease may occur in any bone, but it most commonly involves the medullary cavity of long or tubular bones. Because of the difficulty in separating bone pain during a sickle cell crisis from that of an infection, osteomyelitis can be relatively advanced in these patients by the time the diagnosis is made.
Direct Inoculation Osteomyelitis
This category of osteomyelitis includes infections caused by direct entrance of organisms from a source outside the body. Penetrating wounds (e.g., trauma), open fractures, and various invasive orthopedic procedures can result in direct inoculation of organisms into the bone. More than 80% of cases of postoperative osteomyelitis are known to occur following open reductions of fractures. Specifically, these infections occur most commonly after internal fixation of a hip fracture or femoral or tibial shaft fracture. Osteomyelitis resulting from puncture injuries to the feet are associated with gram-negative infection or the bone and cartilage (sometimes classified as osteochondritis), especially infections caused by P. aeruginosa. S. aureus is also a significant pathogen in these patients.
Contiguous-Spread Osteomyelitis
Osteomyelitis secondary to spread from an adjacent soft tissue infection is called contiguous osteomyelitis. It can result from pressure ulcers or from adjacent soft tissue infections and most often involves the distal extremities. Less commonly, infections can spread from infected teeth to involve the mandible or occur secondary to sinus infections by spreading through the mucosal lining of the sinuses into the vascular system surrounding the bone.16,17
In contrast to hematogenous osteomyelitis, which occurs most commonly in children, contiguous-spread osteomyelitis occurs most commonly in patients older than age 50, most likely because of predisposing factors, such as hip fractures or vascular disease, are more common in this age group.
Contiguous-spread disease has several important differences compared with hematogenous osteomyelitis. Although S. aureus is still the most common organism isolated, infections with multiple organisms, including gram-negative bacilli, occur frequently. P. aeruginosa, streptococcus, E. coli, Staphylococcus epidermidis, and anaerobes can be isolated.
Patients with osteomyelitis in association with severe vascular insufficiency are extremely difficult to manage.18 As anticipated, most of these patients have diabetes mellitus or severe atherosclerosis, and they develop their infections by contiguous spread. Generally, these patients are between the ages of 50 and 70 years. Frequently, patients with vascular disease develop osteomyelitis in their toes and fingers, and there is usually an adjacent area of infection, such as cellulitis or dermal ulcers. Importantly, infections in these patients are almost always polymicrobial and often include staphylococcus and streptococcus or the combination of staphylococcus, streptococcus, and Enterobacteriaceae. Enterococci and anaerobic organisms also can be involved.
Anaerobic organisms also play a role in osteomyelitis. When anaerobes are grown from cultures, they usually are found in association with other organisms, including aerobic bacteria. Predisposing factors in patients who have anaerobic osteomyelitis include vascular disease, bites, contiguous infections, peripheral neuropathy, hematogenous spread, and trauma.19 The anaerobic infections in association with diabetes mellitus almost always occur within the feet. Bacteroides fragilis and Bacteroides melaninogenicus comprise the majority of anaerobic isolates.
Infectious Arthritis
Infectious arthritis usually is acquired by hematogenous spread.6 The synovial tissue is highly vascular and does not have a basement membrane, so organisms in the blood can easily reach the synovial fluid. Table 96–2 summarizes the characteristics of acute infectious arthritis. Some organisms, such as Neisseria gonorrhoeae, are especially likely to infect a joint during bacteremia. Risk factors associated with adult infectious arthritis (more than one factor may be present) are systemic corticosteroid use, preexisting arthritis, arthrocentesis, distant infection, diabetes mellitus, trauma, and other diseases.20
TABLE 96-2 Characteristics of Acute Infectious Arthritis
Organisms also can gain access to the joint from a deep-penetrating wound injury, intraarticular steroid injections, arthroscopy, prosthetic joint surgery, and spread to the joint from a contiguous focus of osteomyelitis. Trauma also appears to be a risk factor in facilitating microbial entry into the synovial space. Unlike children, adults often have significant systemic diseases that predispose them to infectious arthritis, such as diabetes mellitus, immunosuppressive states (e.g., cancer or liver disease), or preexisting arthritis. IV drug abusers and individuals with intravascular infections such as endocarditis also are prone to develop septic arthritis.
Preexisting abnormal joint architecture, joint trauma, and surgery are other important risk factors because chronic inflammation or trauma makes the joint more susceptible to infection. In addition, individuals with rheumatoid arthritis can be prone to bacterial infection because of an inherent phagocytic defect, as well as concomitant corticosteroid therapy. Women are more prone to develop disseminated gonococcal infections than men. The second and third trimesters of pregnancy and the time of menses appear to be the times of greatest risk for developing gonococcal bacteremia.
After bacteria gain access to the joint, the organisms begin to multiply and produce a purulent exudate within the joint. If this joint effusion is present beyond 7 days, chronic, and sometimes irreversible, damage can occur to the bone and joint as a result of proteolytic enzymes and pressure necrosis. Purulent effusions can promote cartilage destruction by increasing leukocyte enzyme activity. In conjunction with the development of the effusion, almost all patients will develop a hot, swollen, painful joint.
![]() S. aureus, the single most common infecting organism, is found in 37% to 65% of cases of nongonococcal bacterial arthritis.7 Streptococcal infections are the second most common and gram-negative organisms are less common. Among the latter, E. coli is the most common; however, P. aeruginosa is the most frequent organism in IV drug abusers. Neonates may have infectious arthritis because of a broad range of organisms, with S. aureus, group B Streptococcus, and gram-negative organisms being most common. S. aureus and streptococcus are the most common pathogens in children younger than 5 years of age. Hib, which used to be the most common pathogen in these children, has essentially been eliminated by immunization with the conjugate Hib vaccine. Pneumococcal arthritis is also decreasing in incidence as a result of conjugate pneumococcal vaccine administration to infants. If a child has not been fully vaccinated or is immunocompromised, Hib may be a cause. Within the adult population, S. aureus is responsible for the vast majority of nongonococcal infections. Gonococcal arthritis is a common manifestation of disseminated gonococcal infection occurring in 42% to 85% of such patients.7 Gonococcal arthritis is now uncommon in North America and Europe although it remains an important concern in developing countries. Although rare, osteomyelitis and infectious arthritis can be caused by fungi and in the case of arthritis by viruses such as varicella-zoster, rubella, or parvovirus.21 Arthritis is rarely caused by Salmonella, Corynebacteria, Brucella, Neisseria meningitides, Mycoplasma pneumoniae, or Ureaplasma urealyticum. Penetrating injury of the joint can result in an infection due to Pasteurella in dog bites, Capnocytophaga in human bites, and Pantoea when the injury is induced by a thorn.
S. aureus, the single most common infecting organism, is found in 37% to 65% of cases of nongonococcal bacterial arthritis.7 Streptococcal infections are the second most common and gram-negative organisms are less common. Among the latter, E. coli is the most common; however, P. aeruginosa is the most frequent organism in IV drug abusers. Neonates may have infectious arthritis because of a broad range of organisms, with S. aureus, group B Streptococcus, and gram-negative organisms being most common. S. aureus and streptococcus are the most common pathogens in children younger than 5 years of age. Hib, which used to be the most common pathogen in these children, has essentially been eliminated by immunization with the conjugate Hib vaccine. Pneumococcal arthritis is also decreasing in incidence as a result of conjugate pneumococcal vaccine administration to infants. If a child has not been fully vaccinated or is immunocompromised, Hib may be a cause. Within the adult population, S. aureus is responsible for the vast majority of nongonococcal infections. Gonococcal arthritis is a common manifestation of disseminated gonococcal infection occurring in 42% to 85% of such patients.7 Gonococcal arthritis is now uncommon in North America and Europe although it remains an important concern in developing countries. Although rare, osteomyelitis and infectious arthritis can be caused by fungi and in the case of arthritis by viruses such as varicella-zoster, rubella, or parvovirus.21 Arthritis is rarely caused by Salmonella, Corynebacteria, Brucella, Neisseria meningitides, Mycoplasma pneumoniae, or Ureaplasma urealyticum. Penetrating injury of the joint can result in an infection due to Pasteurella in dog bites, Capnocytophaga in human bites, and Pantoea when the injury is induced by a thorn.
CLINICAL PRESENTATION
The clinical presentation of acute hematogenous osteomyelitis is summarized in Table 96–3. Although neonatal hematogenous osteomyelitis can spread rapidly to involve the joint, often there are few associated systemic symptoms.22 A joint effusion is present in 60% to 70% of neonatal infections. Decreased limb motion or edema over the affected area may be the only signs from which to suspect the diagnosis. Vertebral osteomyelitis produces nonspecific symptoms, such as constant back pain, fever or night sweats, and weight loss.23 The pain typically is present at rest and increases in severity with movement. Serious neurologic complications can occur if the infection extends and compresses the spinal cord. With contiguous-spread osteomyelitis there is often an area of localized tenderness, warmth, edema, and erythema over the infected site. Patients with significant vascular insufficiency usually have local symptoms, such as pain, swelling, and redness. Less commonly, they also can have fever and elevated white blood cell (WBC) count. The presentation of osteomyelitis after surgery or trauma depends on the precipitating cause. If the infection follows surgery or bone trauma, the symptoms usually are noted within 1 month. The most frequent symptom is pain in the area of infection. Less commonly, patients also can develop a fever and elevated WBC count.
TABLE 96-3 Clinical Presentation of Hematogenous Osteomyelitis
Patients with nongonococcal bacterial arthritis almost always present with a fever, and 50% of patients have an elevated WBC count (see Table 96–2). The average initial synovial WBC count is 10 × 103/mm3 (10 × 109/L) or greater in nongonococcal bacterial disease. The most frequent initial sign of disseminated gonococcal infections is the triad of dermatitis, tenosynovitis, and migratory polyarthralgia or polyarthritis.
Nongonococcal bacterial arthritis is almost always monoarticular. The knee is the most commonly involved joint, but infections also can occur in the shoulder, wrist, hip, ankle, interphalangeal joints, and elbow joints. Usually, the initial focus of infection that acted as the source for bacterial or microbial entrance can be identified. Common routes for bacterial entrance include infections of the respiratory tract, skin, and urinary tract; often no specific source can be identified. Blood cultures are important in these patients because they can be positive in 50% of patients.
Another type of infectious arthritis occurs following prosthetic joint surgery. The most common symptom is pain. Local signs of inflammation and fever are common in acute infections while chronic infections present in a more subtle fashion, typically with pain alone and often loosening of the prosthesis. With these infections, the C-reactive protein usually is elevated, although a leukocytosis often is absent. Infections that result from postoperative contamination usually become apparent within 1 year of surgery.
Radiologic and Laboratory Tests
The evaluation of a patient who may have osteomyelitis has several unusual aspects. Radiographs of the involved area should be obtained to rule out other processes such as a fracture; bone changes characteristic of osteomyelitis appear late and are not typically seen until at least 10 to 14 days after the onset of the infection as more than 50% of the bone matrix must be removed before the lesions can be detected radiologically. Magnetic resonance imaging is the most sensitive and commonly used diagnostic imaging modality and offers the advantage of better anatomic definition, especially of abscesses or joint effusions. Radionuclide bone scanning is useful in identifying the focus of osteomyelitis.24,25
Despite the seriousness of osteomyelitis, often there are few laboratory abnormalities. The erythrocyte sedimentation rate (ESR), C-reactive protein, and WBC count may be the only laboratory abnormalities.11 The degree of abnormality of these laboratory findings does not correlate with the disease outcome; however, they are useful for monitoring therapy. C-reactive protein can be elevated because of the presence of inflammation, and it can be substituted for the ESR. C-reactive protein is generally the more sensitive marker of response to therapy and often increases and decreases before the ESR.
When a clinical assessment of osteomyelitis is suspected, it is important to establish a bacteriologic diagnosis by culture of the infected bone. Accurate culture information is especially important as a guide for treatment of osteomyelitis in this era of increasing antimicrobial resistance. Bone aspiration and bone biopsy are valuable in determining an accurate bacteriologic diagnosis. In addition, they help determine whether or not there is an abscess present. If an abscess is identified, it must be drained and the pus is cultured, and a Gram stain is performed. Aspirates of subperiosteal pus or metaphyseal fluid yield a pathogen in 70% of cases. Cultures should be done for both aerobic and anaerobic bacteria. A Gram stain of the aspirate can be useful in initiating appropriate empirical antibiotic therapy.
If a specimen is obtained from a previously undrained or unopened wound abscess, the pathogen usually can be identified. In chronic osteomyelitis, however, identification can be more difficult.26 Open wounds and draining sinuses frequently are contaminated with other organisms and thus provide inaccurate culture information.27 They cannot be relied on to reflect the pathogen unless consecutive deep sinus tract cultures reveal the same pathogens.28 Cultures of loculated pus aspirates in the area of orthopedic devices removed from infected bone can be trusted, however, to identify the infecting organism. In diabetic patients that may have osteomyelitis, bone infections are most common in patients with foot ulcers greater than 3 mm and in patients with C-reactive protein levels greater than 3.2 mg/dL (32 mg/L).29 The preferable time to obtain culture material in a patient with a chronic draining sinus is at the time of open surgical debridement.
In addition to performing cultures from the involved bone, it also is important to obtain cultures from any site believed to be the source of a bacteremia. Blood cultures should be obtained. Approximately 50% of patients with hematogenous osteomyelitis will have positive blood cultures and may obviate the need for bone aspiration in these patients.
![]() When evaluating the possibility of a patient having infectious arthritis, immediate joint aspiration with subsequent analysis of the synovial fluid is extremely important. The presence of purulent fluid usually indicates the presence of a septic joint. The synovial fluid WBC count is usually 50 to 200 × 103/mm3 (50 × 109 to 200 × 109/L) when an infection is present. However, serum WBC, ESR, and C-reactive protein may not be useful acutely in septic arthritis.30 Approximately half the patients with an infected joint have a low synovial glucose level, usually less than 40 mg/dL (2.2 mmol/L). Gram stains of joint fluid demonstrate bacteria in 50% of patients with septic arthritis; however, such stains are positive in only 25% of patients with gonococcal arthritis. Synovial fluid cultures usually are positive in patients with nongonococcal infections. Both blood and joint fluid should be cultured aerobically and anaerobically in a patient suspected of having an infected joint. Blood cultures are positive in one half of patients with nongonococcal infections but in only 20% of those with gonococcal infections. Pharyngeal, rectal, cervical, or urethral smears and cultures, as well as cultures of cutaneous lesions, should be performed if a disseminated gonococcal infection is considered. As with osteomyelitis, most patients will have an elevated C-reactive protein concentration and ESR. Radiographs of infected joints often reveal distension of the joint capsule with soft tissue swelling in the adjacent space. Magnetic resonance imaging can be helpful in identifying an infected joint, especially the hip. In patients who have developed an infected prosthetic joint, loosening of the prosthesis can be seen radiographically.
When evaluating the possibility of a patient having infectious arthritis, immediate joint aspiration with subsequent analysis of the synovial fluid is extremely important. The presence of purulent fluid usually indicates the presence of a septic joint. The synovial fluid WBC count is usually 50 to 200 × 103/mm3 (50 × 109 to 200 × 109/L) when an infection is present. However, serum WBC, ESR, and C-reactive protein may not be useful acutely in septic arthritis.30 Approximately half the patients with an infected joint have a low synovial glucose level, usually less than 40 mg/dL (2.2 mmol/L). Gram stains of joint fluid demonstrate bacteria in 50% of patients with septic arthritis; however, such stains are positive in only 25% of patients with gonococcal arthritis. Synovial fluid cultures usually are positive in patients with nongonococcal infections. Both blood and joint fluid should be cultured aerobically and anaerobically in a patient suspected of having an infected joint. Blood cultures are positive in one half of patients with nongonococcal infections but in only 20% of those with gonococcal infections. Pharyngeal, rectal, cervical, or urethral smears and cultures, as well as cultures of cutaneous lesions, should be performed if a disseminated gonococcal infection is considered. As with osteomyelitis, most patients will have an elevated C-reactive protein concentration and ESR. Radiographs of infected joints often reveal distension of the joint capsule with soft tissue swelling in the adjacent space. Magnetic resonance imaging can be helpful in identifying an infected joint, especially the hip. In patients who have developed an infected prosthetic joint, loosening of the prosthesis can be seen radiographically.
TREATMENT
Desired Outcome
The goals of treatment are resolution of the infection and prevention of long-term sequelae. The ultimate outcome of osteomyelitis depends on the acute or chronic nature of the disease and how rapidly appropriate therapy is initiated. Patients with acute osteomyelitis have the best prognosis. Cure rates exceeding 80% can be expected for patients with acute osteomyelitis who have surgery when indicated and receive appropriate antibiotics for 4 to 6 weeks. When the growth plate is involved in children, discrepancies in the growth of bones or angular bone deformities can result.
In contrast, patients with chronic osteomyelitis have a much poorer prognosis. Dead bone and other necrotic material from the infection act as a bacterial reservoir and make the infection very difficult to eliminate. Adequate surgical debridement to remove all the dead bone and necrotic material, combined with prolonged administration of antibiotics, provides the best chance to obtain a cure.31 The inability to remove all the dead bone can allow residual infection and require suppressive antibiotics to control the infection.
While many patients who develop infectious arthritis recover with no long-term sequelae, 50% are left with decreased joint function or mobility. Gonococcal arthritis usually resolves rapidly with antibiotics and has a lower rate of sequelae. Individuals at greatest risk for long-term sequelae are those who have symptoms present for more than 7 days before starting therapy and those with infections occurring within the hip joint and infections caused by gram-negative organisms. Common long-term residual effects following infectious arthritis are limited joint motion and persistent pain.
General Approach to Treatment
![]() Following completion of the steps needed to determine the infecting organism, the most important treatment modality of acute osteomyelitis is the administration of appropriate antibiotics in adequate doses for a sufficient length of time. It is important to stress that early antibiotic therapy can mitigate the need for surgery, subsequent sepsis, chronic infection, disruption of longitudinal bone growth, and angular deformity of the bone.32 A delay in treatment can allow bone necrosis to occur and make eradication of the infection much more difficult. In these patients with chronic osteomyelitis, exacerbations of the infection can result if all necrotic tissue is not removed surgically and all microorganisms eliminated. Chronic suppressive antimicrobial therapy and adjunctive treatment with hyperbaric oxygen or antibiotic-impregnated implants during surgery also has been used.33,34
Following completion of the steps needed to determine the infecting organism, the most important treatment modality of acute osteomyelitis is the administration of appropriate antibiotics in adequate doses for a sufficient length of time. It is important to stress that early antibiotic therapy can mitigate the need for surgery, subsequent sepsis, chronic infection, disruption of longitudinal bone growth, and angular deformity of the bone.32 A delay in treatment can allow bone necrosis to occur and make eradication of the infection much more difficult. In these patients with chronic osteomyelitis, exacerbations of the infection can result if all necrotic tissue is not removed surgically and all microorganisms eliminated. Chronic suppressive antimicrobial therapy and adjunctive treatment with hyperbaric oxygen or antibiotic-impregnated implants during surgery also has been used.33,34
If a patient with hematogenous osteomyelitis does not respond by having a decrease in fever, local swelling, redness, and pain following the initiation of adequate antibiotic therapy, the patient should undergo surgical debridement of the infected area. It is important to emphasize the priority of starting antibiotics immediately after the cultures have been obtained.
Pharmacologic Therapy
Antibiotic Bone Concentration
![]() Antibiotics used in the management of acute osteomyelitis generally are given in high doses (adjusted for weight, renal function, hepatic function, or both) so that adequate antimicrobial concentrations are reached within the infected bone and joint.35 Between 8 and 12 g/day of a penicillinase-resistant penicillin (nafcillin or oxacillin), ampicillin, or cephalosporin or a similar large dose of another parenteral antibiotic is used in the initial management of adults with osteomyelitis.36 These dosing recommendations, however, are empirical; the relationship between a specific dose of a given antibiotic and its resulting concentration within the infected bone is largely unknown. Semisynthetic penicillins, cephalosporins, clindamycin, and the aminoglycosides can be detected in bone homogenates soon after their administration.
Antibiotics used in the management of acute osteomyelitis generally are given in high doses (adjusted for weight, renal function, hepatic function, or both) so that adequate antimicrobial concentrations are reached within the infected bone and joint.35 Between 8 and 12 g/day of a penicillinase-resistant penicillin (nafcillin or oxacillin), ampicillin, or cephalosporin or a similar large dose of another parenteral antibiotic is used in the initial management of adults with osteomyelitis.36 These dosing recommendations, however, are empirical; the relationship between a specific dose of a given antibiotic and its resulting concentration within the infected bone is largely unknown. Semisynthetic penicillins, cephalosporins, clindamycin, and the aminoglycosides can be detected in bone homogenates soon after their administration.
Daptomycin may also be an effective empiric therapy for the treatment of osteomyelitis in adults caused by most methicillin-susceptible and methicillin-resistant S. aureus (MRSA), but the data are limited.37 Further prospective studies are needed to define the situations in which daptomycin might be best utilized and its optimal dosing.



