BONE GROWTH, REMODELING, & REPAIR
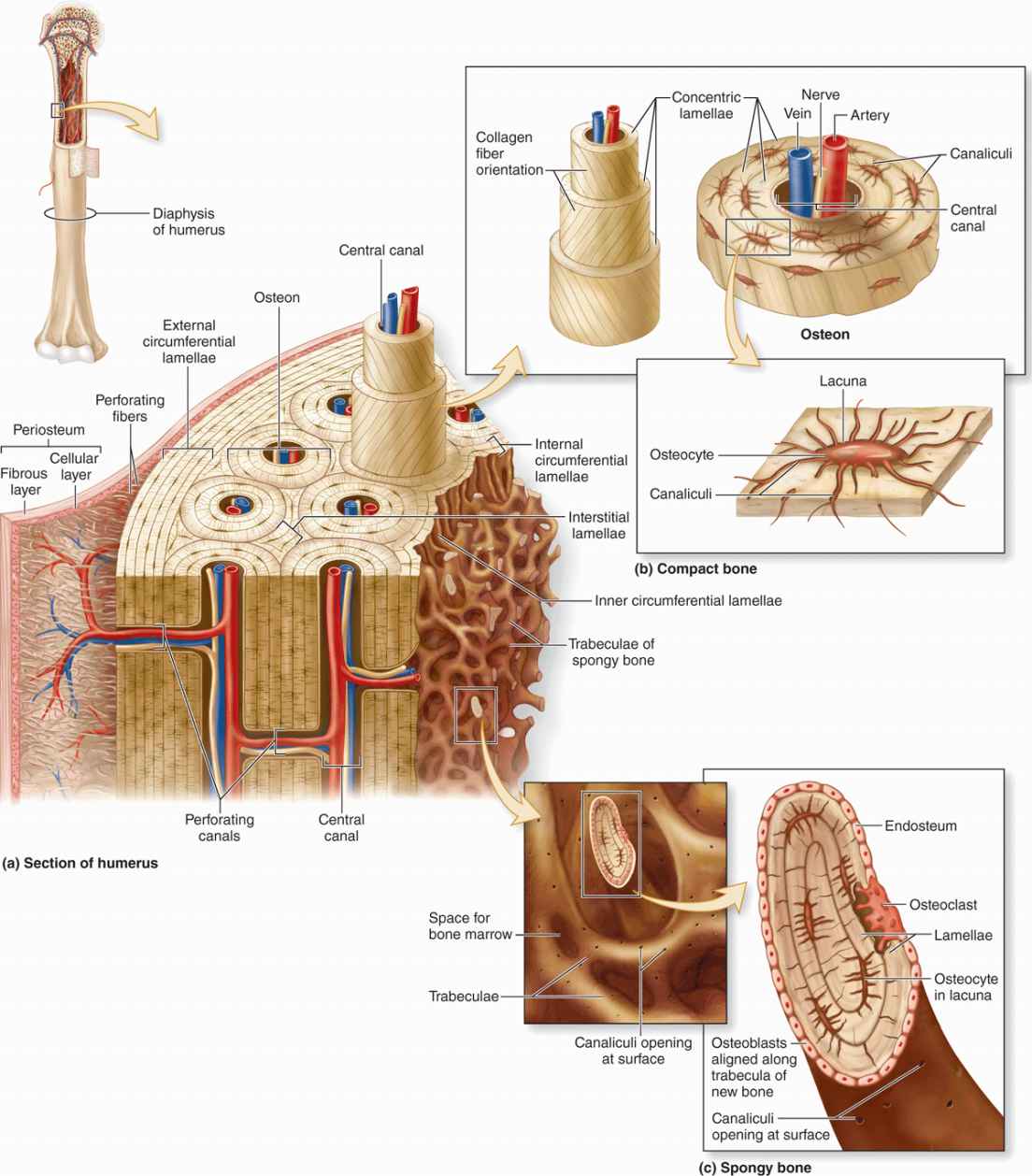
As the main constituent of the adult skeleton, bone tissue (Figure 8–1) provides solid support for the body, protects vital organs such as those in the cranial and thoracic cavities, and harbors cavities containing bone marrow where blood cells are formed. Bone (or osseous) tissue also serves as a reservoir of calcium, phosphate, and other ions that can be released or stored in a controlled fashion to maintain constant concentrations in body fluids.
FIGURE 8–1 Components of bone.
In addition, bones form a system of levers that multiply the forces generated during skeletal muscle contraction and transform them into bodily movements. This mineralized tissue therefore confers mechanical and metabolic functions to the skeleton.
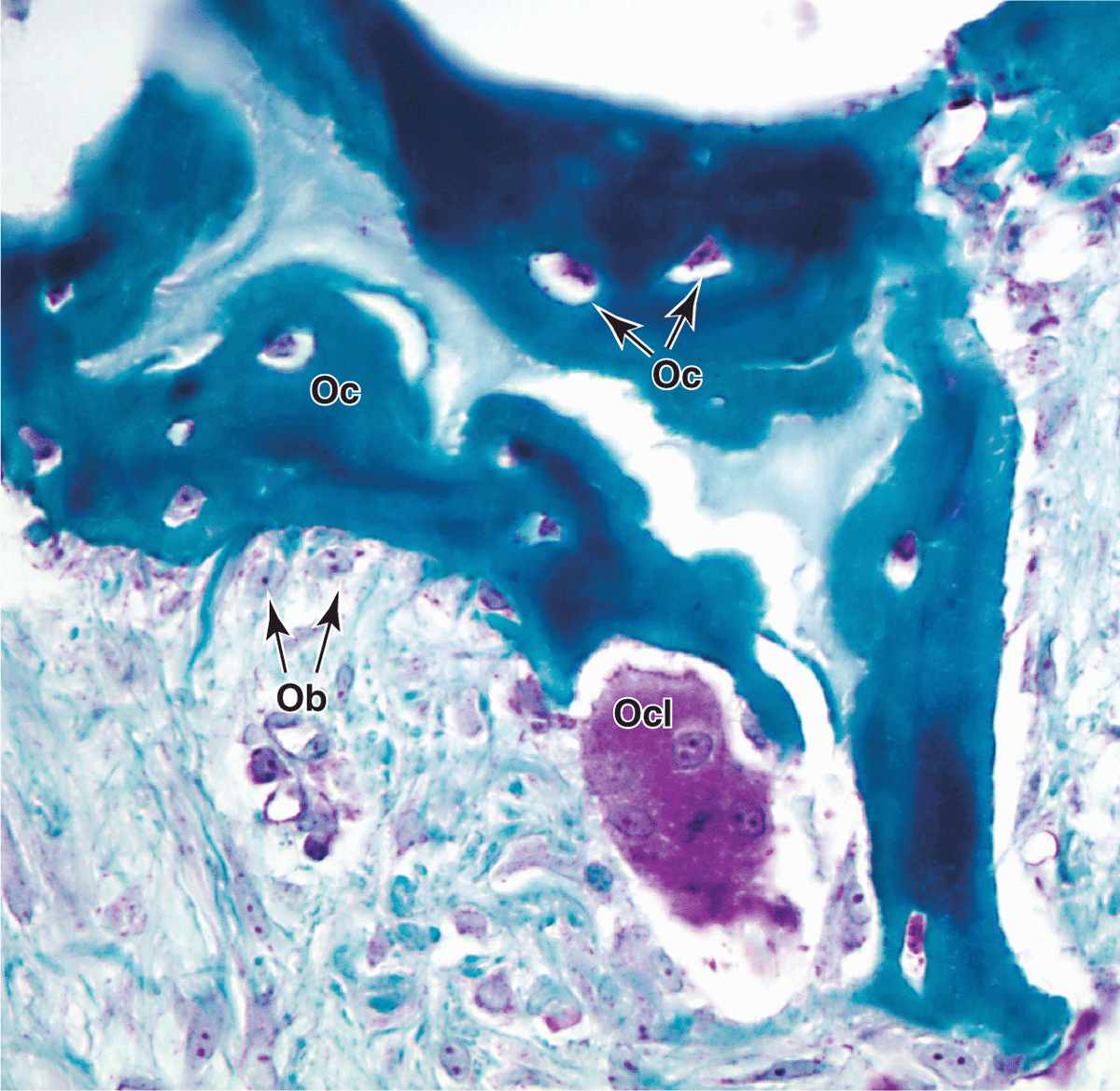
Bone is a specialized connective tissue composed of calcified extracellular material, the bone matrix, and three major cell types (Figure 8–2):
FIGURE 8–2 Osteoblasts, osteocytes, and an osteoclast.
 Osteocytes (Gr. osteon, bone + kytos, cell), which are found in cavities (lacunae) between bone matrix layers (lamellae), with cytoplasmic processes extending into small canaliculi (L. canalis, canal) between lamellae (Figure 8–1).
Osteocytes (Gr. osteon, bone + kytos, cell), which are found in cavities (lacunae) between bone matrix layers (lamellae), with cytoplasmic processes extending into small canaliculi (L. canalis, canal) between lamellae (Figure 8–1).
 Osteoblasts (osteon + Gr. blastos, germ), which synthesize the organic components of the matrix
Osteoblasts (osteon + Gr. blastos, germ), which synthesize the organic components of the matrix
 Osteoclasts (osteon + Gr. klastos, broken), which are multinucleated, giant cells involved in the resorption and remodeling of bone tissue.
Osteoclasts (osteon + Gr. klastos, broken), which are multinucleated, giant cells involved in the resorption and remodeling of bone tissue.
Because metabolites are unable to diffuse through the calcified matrix of bone, the exchanges between osteocytes and blood capillaries depend on communication through the very thin, cylindrical spaces of the canaliculi.
All bones are lined on both internal and external surfaces by layers of connective tissue containing osteogenic cells—endosteum on the internal surface surrounding the marrow cavity and periosteum on the external surface.
Because of its hardness, bone cannot be sectioned routinely. Bone matrix is usually softened by immersion in a decalcifying solution before paraffin embedding, or embedded in plastic after fixation and sectioned with a specialized microtome.
BONE CELLS
Osteoblasts
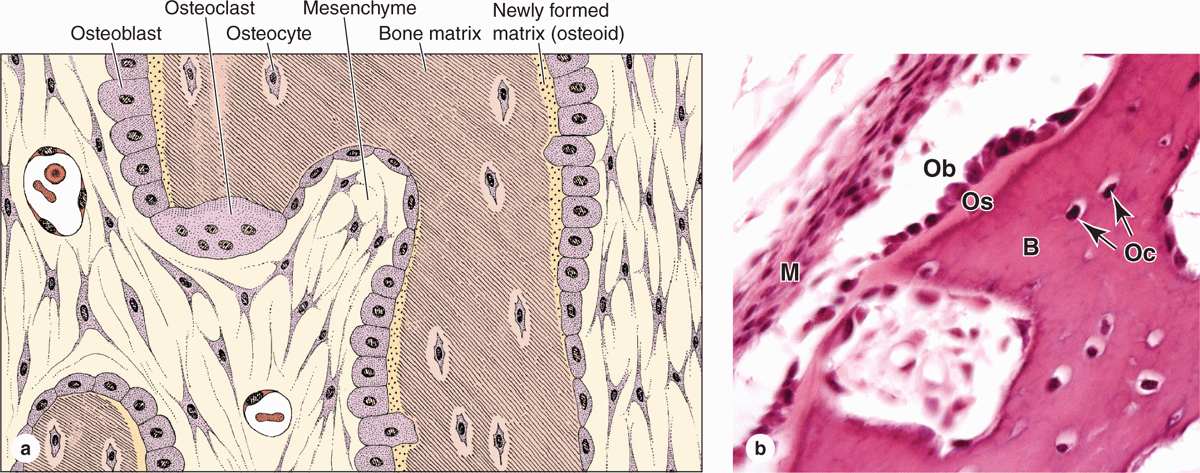
Osteoblasts synthesize and secrete the organic components of bone matrix, which include type I collagen fibers, proteoglycans, and several glycoproteins such as osteonectin. Deposition of the inorganic components of bone also depends on viable osteoblasts. Mature osteoblasts are located exclusively at the surfaces of bone matrix, usually side by side in a layer somewhat resembling a simple epithelium (Figures 8–2 and 8–3). When actively engaged in matrix synthesis, osteoblasts have a cuboidal to columnar shape and basophilic cytoplasm. When their synthesizing activity declines, they flatten and basophilia is reduced; inactive osteoblasts represent most of the flattened bone lining cells in both the endosteum and periosteum.
FIGURE 8–3 Osteoblasts and osteocytes.
During matrix synthesis, osteoblasts have the ultrastructure of cells actively synthesizing proteins for secretion. Osteoblasts are polarized cells: matrix components are secreted at the cell surface in contact with existing bone matrix, producing a layer of new (but not yet calcified) material called osteoid between the osteoblast layer and the preexisting bone surface (Figure 8–2). This process of bone appositional growth is completed by subsequent deposition of calcium salts into the newly formed matrix.
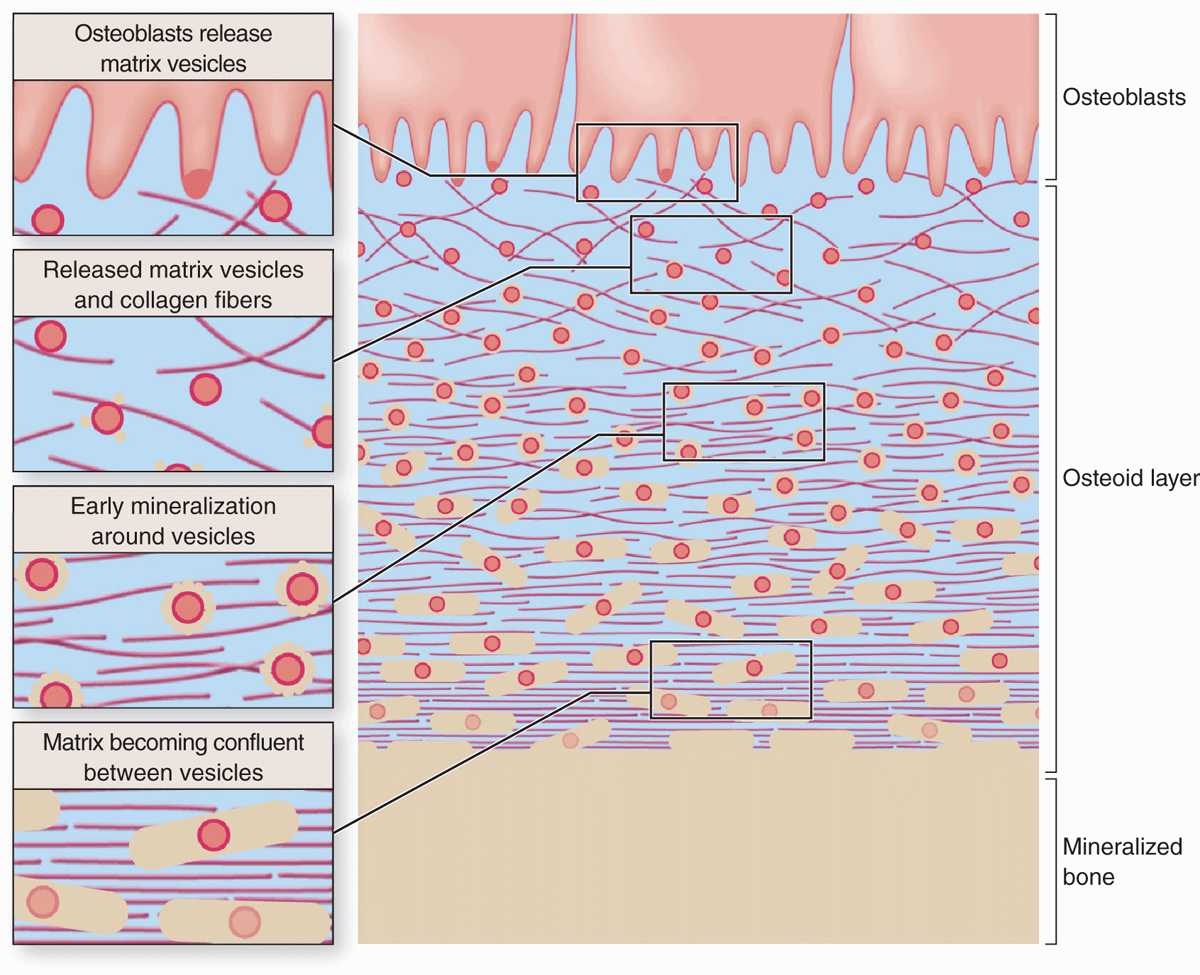
Calcification of the matrix is not completely understood, but basic aspects of the process are shown in Figure 8–4. Prominent among the noncollagen proteins secreted by osteoblasts is the small, vitamin K-dependent polypeptide osteocalcin, which together with various glycoproteins binds Ca2+ ions and raises their concentration locally. Osteoblasts also release membrane-enclosed matrix vesicles rich in alkaline phosphatase and other enzymes whose activity raises the local concentration of PO4– ions. With high concentrations of both calcium and phosphate ions, these vesicles serve as foci for the formation of hydroxyapatite [Ca10(PO4)6(OH)2] crystals, the first visible step in calcification. These crystals grow rapidly by accretion of more mineral and eventually produce a confluent mass of calcified material embedding the collagen fibers and proteoglycans.
FIGURE 8–4 Mineralization in bone matrix.
Osteocytes
Many osteoblasts are gradually surrounded by the material they secreted and differentiate further as osteocytes enclosed singly within the lacunae that are regularly spaced throughout the mineralized matrix. In the transition from osteoblasts to osteocytes, the cells extend many long dendritic processes, which also become surrounded by calcifying matrix. Osteocytic processes thus come to occupy the many canaliculi, 250-300 nm in diameter, that radiate from each lacuna (Figure 8–5; see Figure 8–1).
FIGURE 8–5 Osteocytes in lacunae.
Diffusion of metabolites between osteocytes and blood vessels occurs through the small amount of extracellular fluid between the bone matrix and the osteocytes and their processes. Osteocytes also communicate with one another via gap junctions on the dendritic processes in the canaliculi and on osteoblasts and bone lining cells.
When compared with osteoblasts, the flat, almond-shaped osteocytes exhibit significantly less RER, smaller Golgi complexes, and more condensed nuclear chromatin (Figure 8–5a). These cells maintain the bony matrix, and their death is followed by rapid matrix resorption. Osteocytes express a different array of genes compared to osteoblasts, and osteocyte products such as the protein sclerostin and certain cytokines help regulate bone remodeling. The extensive lacunar-canalicular network of osteocytes and their communication with all other bone cells suggest additional roles for osteocytes in calcium homeostasis and as sensors for detection of mechanical stresses on bone, which is also important in directing bone remodeling.
Osteoclasts
Osteoclasts are very large, motile cells with multiple nuclei (Figure 8–6) and play a major role in matrix resorption during bone growth and remodeling. The large size and multinucleated condition of osteoclasts are due to their origin from the fusion of bone marrow-derived cells. Osteoclast development requires two polypeptides produced by osteoblasts: macrophage-colony-stimulating factor (M-CSF; discussed with hemopoiesis, Chapter 13) and the receptor activator of nuclear factor-κB ligand (RANKL). In areas of bone undergoing resorption, osteoclasts lie within enzymatically etched depressions or cavities in the matrix known as resorption cavities (also called Howship lacunae).
FIGURE 8–6 Osteoclasts and their activity
In active osteoclasts, the surface against the bone matrix is folded into irregular projections, forming a ruffled border surrounded by a cytoplasmic zone rich in actin filaments, which is the site of adhesion to the matrix. This circumferential adhesion zone creates a microenvironment between the osteoclast and the matrix in which bone resorption occurs (see Figure 8–6b).
Into this subcellular pocket the osteoclast secretes collagenase, cathepsin K, and other enzymes and pumps protons to produce an acidic environment locally for dissolving hydroxyapatite and promoting the localized digestion of matrix proteins. Osteoclast activity is controlled by local signaling factors and hormones. Osteoclasts have receptors for calcitonin, a thyroid hormone. Osteoblasts activated by parathyroid hormone (PTH) produce M-CSF, RANKL, and other factors that regulate the formation and activity of osteoclasts.
BONE MATRIX
Inorganic material represents about 50% of the dry weight of bone matrix. Calcium hydroxyapatite is most abundant, but bicarbonate, citrate, magnesium, potassium, and sodium ions are also found. Significant quantities of amorphous (noncrystalline) calcium phosphate are also present. The surface ions of hydroxyapatite crystals are hydrated; the layer of water around the crystal facilitates the exchange of ions between the mineral and body fluids.
The organic matter embedded in the calcified matrix includes type I collagen, proteoglycan aggregates, and bonespecific multiadhesive glycoproteins such as osteonectin. Calcium-binding glycoproteins, notably osteocalcin, and the phosphatases released in matrix vesicles by osteoblasts promote calcification of the matrix. Other tissues containing type I collagen do not contain osteocalcin or matrix vesicles and are not normally calcified. Because of its high collagen content, decalcified bone matrix is usually acidophilic.
The association of minerals with collagen fibers during calcification is responsible for the hardness and resistance of bone tissue. After a bone is decalcified, its shape is preserved, but it becomes as flexible as a tendon.
PERIOSTEUM & ENDOSTEUM
External and internal surfaces of bone are covered by tissue layers with bone-forming cells, called periosteum and endosteum, respectively.
The periosteum is organized much like the perichondrium (see Figure 7–3a). The outer layer is dense connective tissue, with small blood vessels, collagen bundles, and fibroblasts (see Figure 8–1). Bundles of periosteal collagen fibers, called perforating (or Sharpey) fibers, penetrate the bone matrix, binding the periosteum to bone. The inner region of periosteum is a more cellular layer containing bone lining cells, osteoblasts, and mesenchymal stem cells called osteoprogenitor cells. With the potential to proliferate and differentiate into osteoblasts, osteoprogenitor cells play a prominent role in bone growth and in bone repair. The principal functions of periosteum are to nourish the osseous tissue and provide a continuous supply of new osteoblasts for appositional bone growth or repair.
Internally the very thin endosteum covers small trabeculae of bony matrix that project into the marrow cavities (Figures 8–1 and 8–3). Although considerably thinner than the periosteum, endosteum also contains osteoprogenitor cells, osteoblasts, and bone lining cells.
TYPES OF BONE
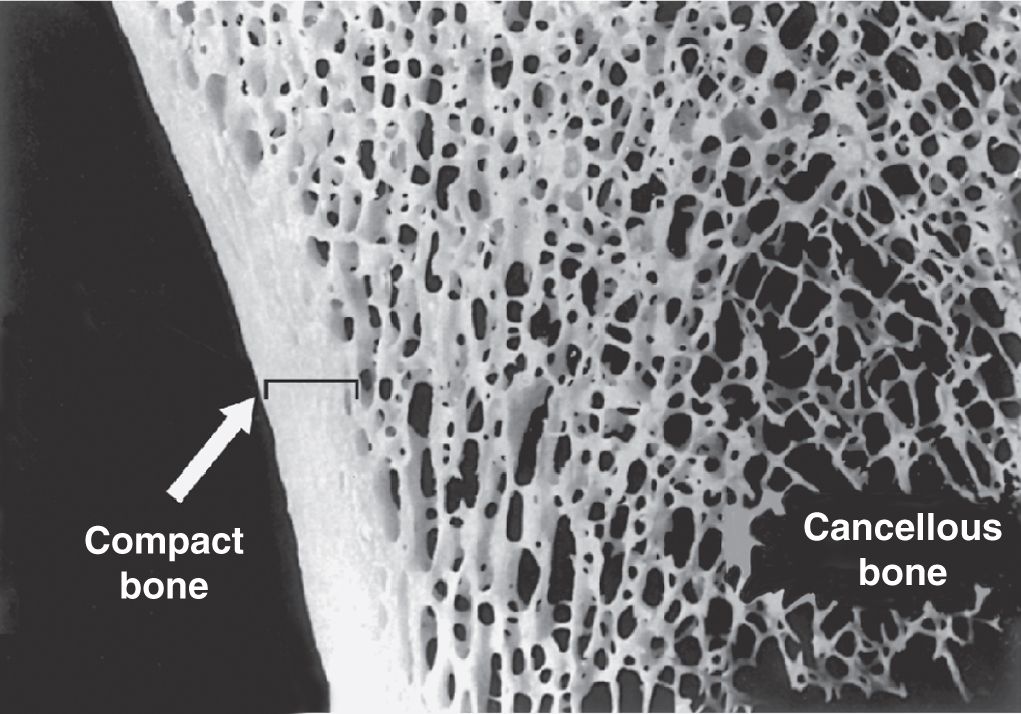
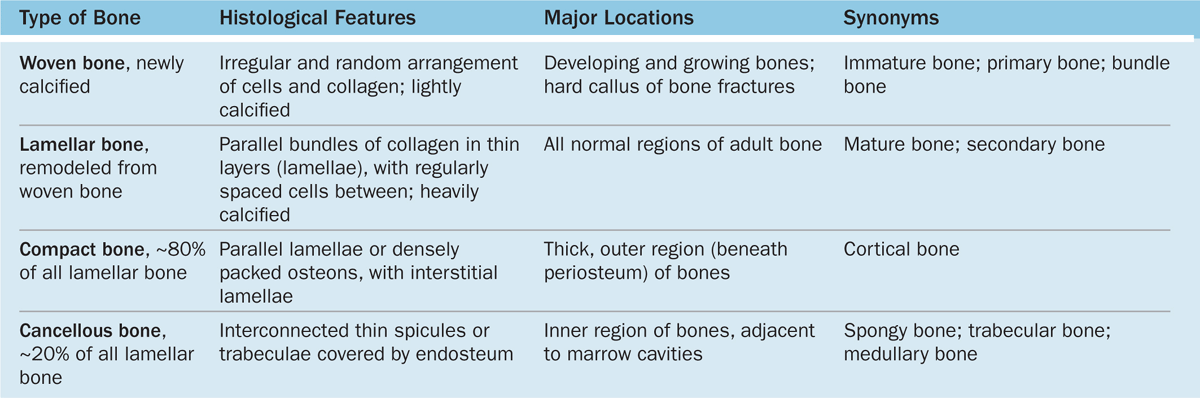
Gross observation of a bone in cross section (Figure 8–7) shows a dense area near the surface corresponding to compact (cortical) bone, which represents 80% of the total bone mass, and deeper areas with numerous interconnecting cavities, called cancellous (trabecular or spongy) bone, constituting about 20% of total bone mass. Histological features and important locations of the major types of bone are summarized (Table 8–1) near the end of this chapter.
FIGURE 8–7 Compact and cancellous bone.
TABLE 8–1 Summary of bone types and their organization.
In long bones, the bulbous ends—called epiphyses (Gr. epiphysis, an excrescence)—are composed of spongy bone covered by a thin layer of compact bone. The cylindrical part—the diaphysis (Gr. diaphysis, a growing between)—is almost totally composed of compact bone, with a thin region of spongy bone on the inner surface around the central marrow cavity (see Figure 8–1). Short bones such as those of the wrist and ankle usually have cores of spongy bone surrounded completely by compact bone. The flat bones that form the calvaria (skullcap) have two layers of compact bone called plates, separated by a thicker layer of spongy bone called the diploë.
Microscopic examination of bone tissue shows two types of organization: lamellar bone and woven bone, which is usually more immature than lamellar bone.
Lamellar Bone
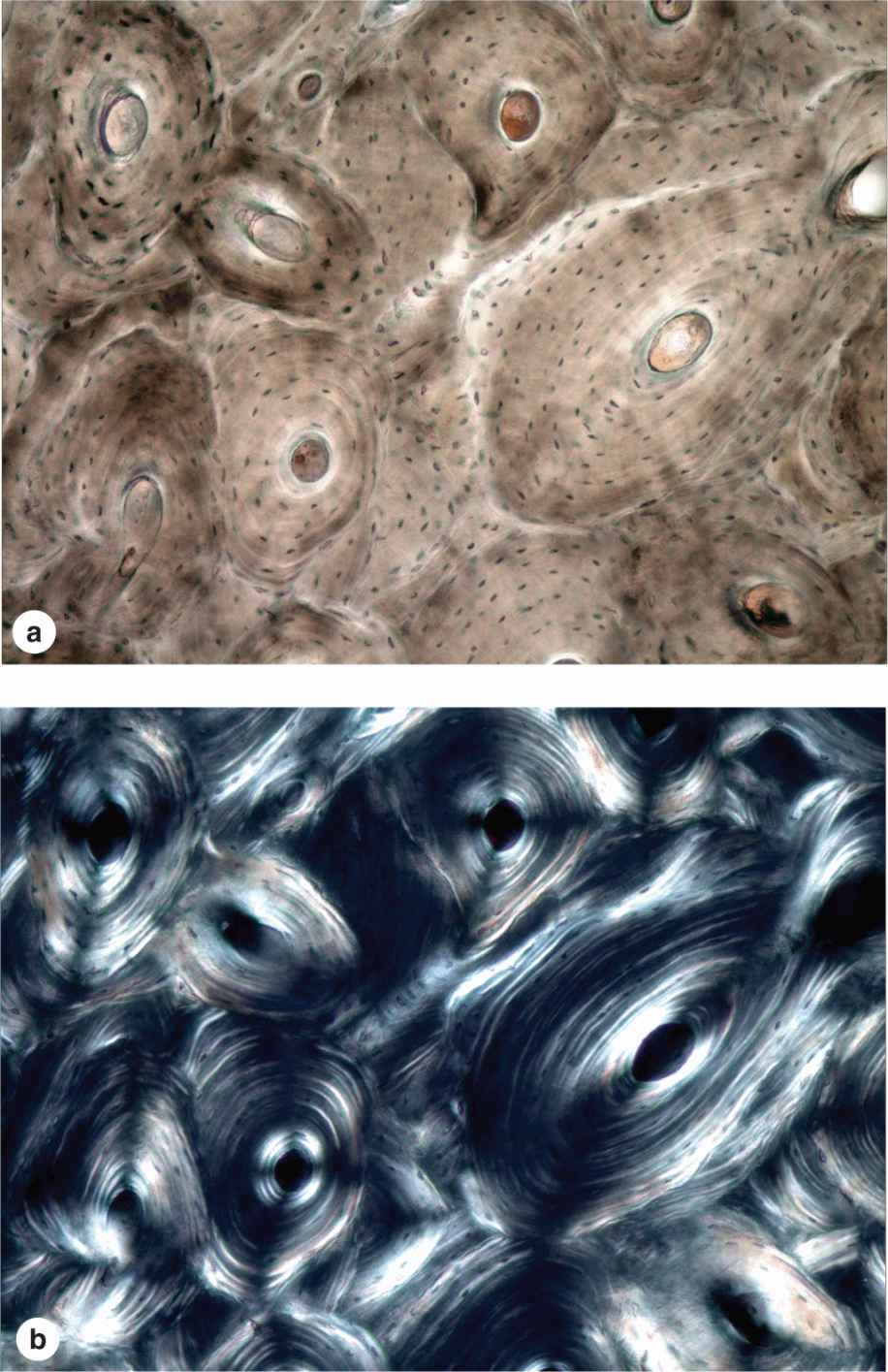
Most bone in adults, compact or cancellous, is organized as lamellar bone, characterized by multiple layers or lamellae of calcified matrix, each 3-7 μm thick. The lamellae are organized either parallel to each other or concentrically around a central canal. In each lamella, type I collagen fibers are aligned in parallel, with the pitch of the fibers’ orientation shifted orthogonally (by about 90 degrees) in successive lamellae. This highly ordered organization of collagen within lamellar bone is visible under the polarizing light microscope as birefringence; alternating bright and dark layers are due to the changing orientation of collagen fibers in the lamellae (Figure 8–8). Like the orientation of wood fibers in plywood, the highly ordered organization of collagen fibers in lamellae adds greatly to the strength of lamellar bone.
FIGURE 8–8 Lamellar bone.
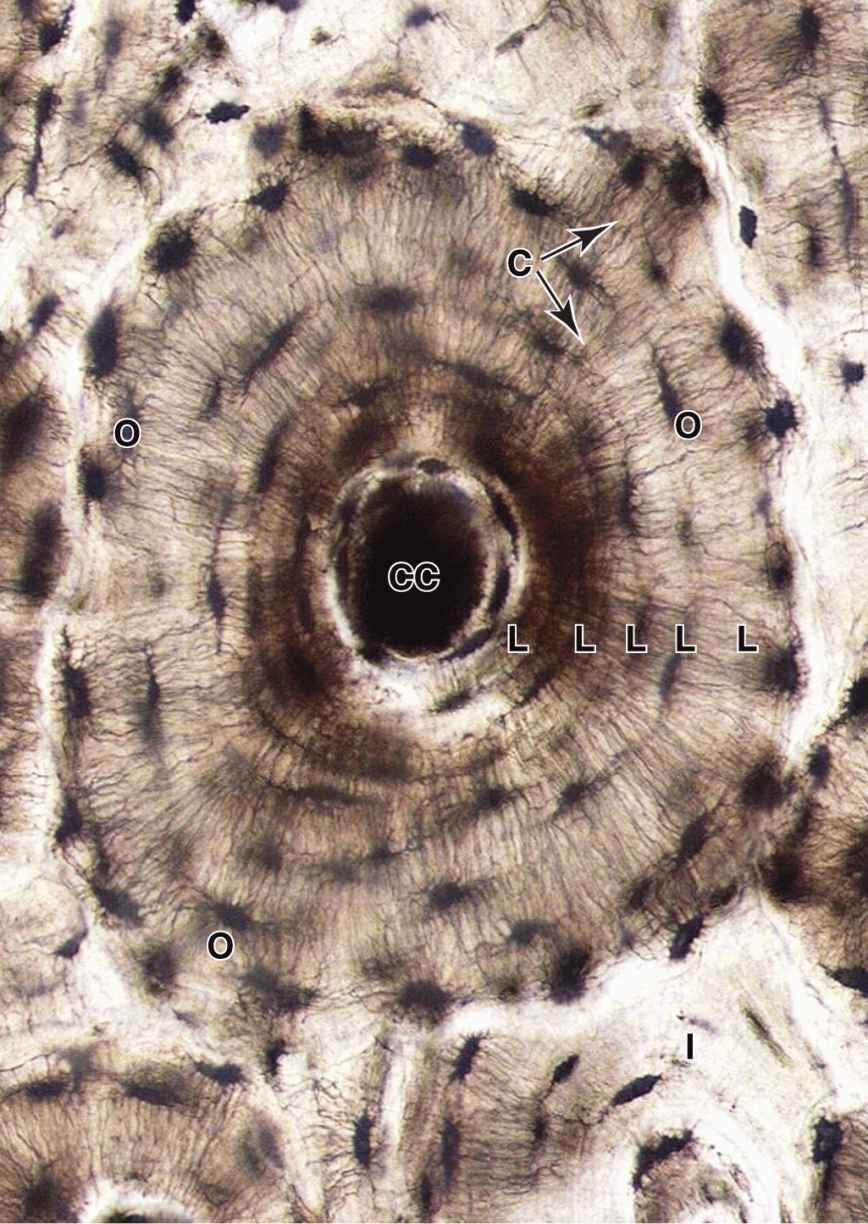
An osteon (or Haversian system) refers to the complex of concentric lamellae surrounding a small central canal that contains blood vessels, nerves, loose connective tissue, and endosteum (see Figures 8–1 and 8–9). Between successive lamellae are lacunae, each with one osteocyte, interconnected by canaliculi containing the cells’ dendritic processes (Figure 8–9). Processes of adjacent cells are in contact via gap junctions, and all cells of an osteon receive nutrients and oxygen from the microvasculature in the central canal (see Figure 8–1). The outer boundary of each osteon is a more collagen-rich layer called the cement line.
FIGURE 8–9 An osteon.
 MEDICAL APPLICATION
MEDICAL APPLICATION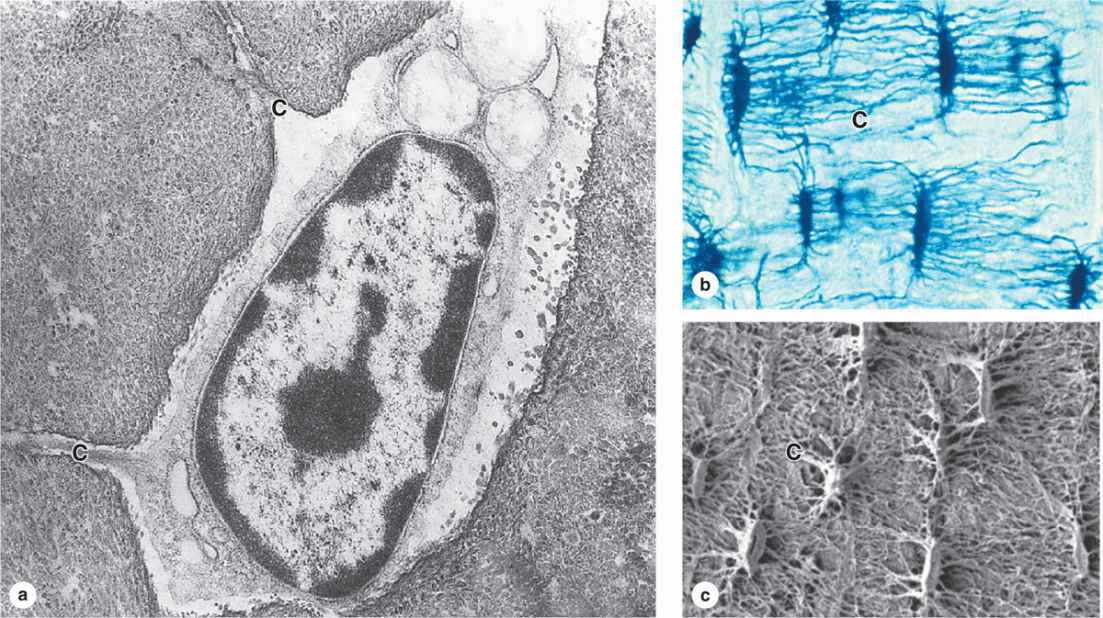
 MEDICAL APPLICATION
MEDICAL APPLICATION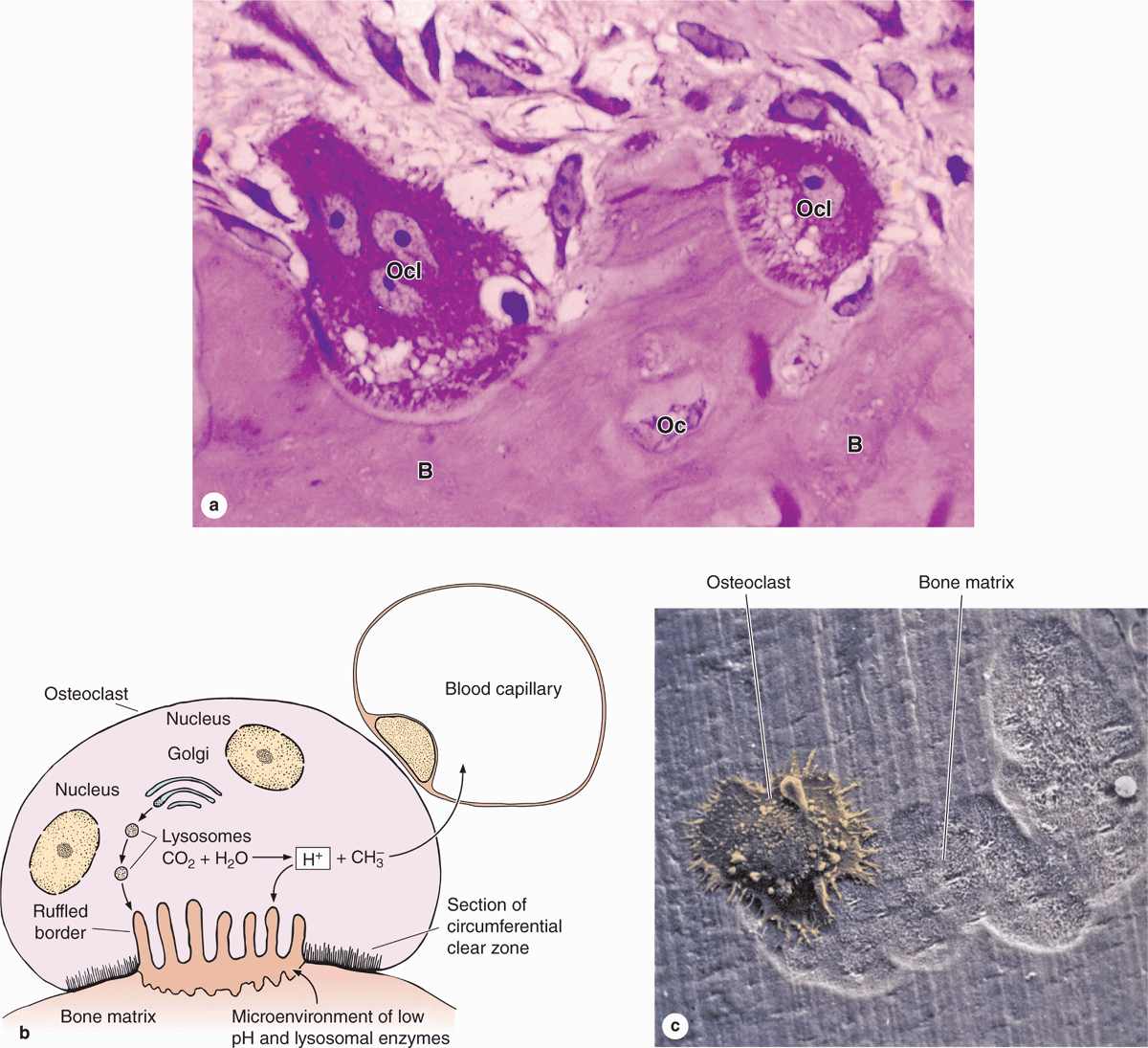
 MEDICAL APPLICATION
MEDICAL APPLICATION MEDICAL APPLICATION
MEDICAL APPLICATION