Chapter 3 Bonds found in biological chemistry
Each electron orbital has a specific allocation of spaces available for electrons to fill (see Figure 2.3, p. 9). In the case of the outer orbitals, it is unusual to find all the spaces empty or full and one atom will seek out another compatible atom so that they can share their respective electrons and fill their outer orbitals. This arrangement creates great stability for the associated atoms, which is why, whenever possible, bonds are formed, between individual atoms.
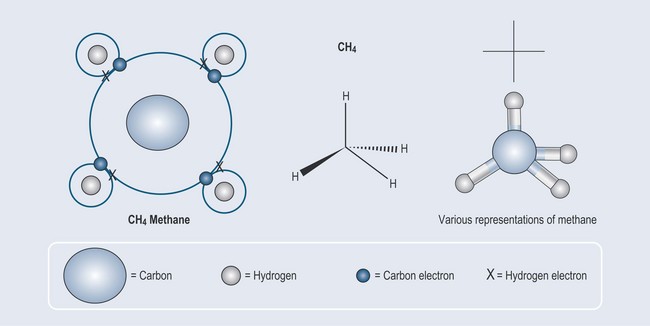
Taking the examples of hydrogen and carbon used in Chapter 2 (Figure 2.3), it is possible to see that hydrogen:
The final result of this union is that four hydrogen atoms attach themselves to one carbon atom, thus filling the outer orbitals of both the carbon and the hydrogens satisfying the needs of both elements (Figure 3.1).
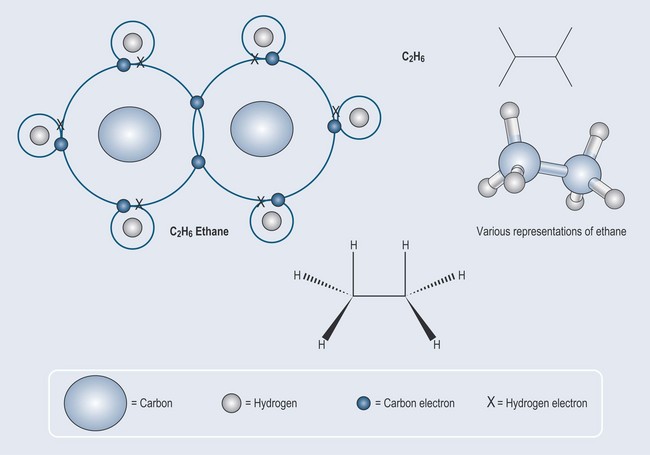
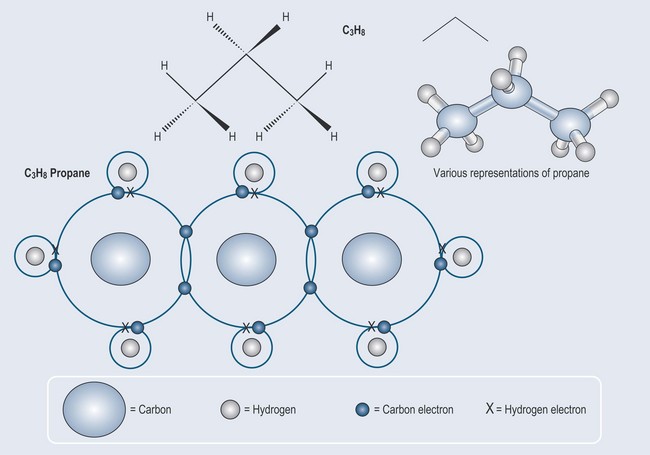
It is a matter of simple arithmetic to work out where this leads: a more complex molecule – ethane – can be formed from two carbon atoms and six hydrogen atoms Figure 3.2. The process can be continued to make an even longer compound called propane (Figure 3.3).
Note: as drawing chemicals out in this way can become complicated, there is a need for a method of abbreviation. Each figure, therefore, contains examples of various chemical shorthands used to represent these structures. This will enable you to become used to seeing them. Naming and representation will be covered in more detail in Chapter 5 ‘Nomenclature: representation of chemical structures and basic terminology’.
Covalent Bonds
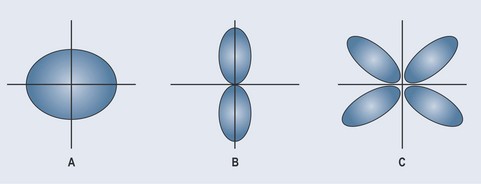
For a greater appreciation, blow up balloons of different shapes and arrange them as you see them in Figure 3.4. The different balloons are the accepted shapes of electron orbitals and the shape of the electrostatic force created by this orbital. Notice how it is possible to bend the orbital shown in B around the axis, but that in C the movement around the axis is restricted because the sides of the balloons push against one another limiting movement.
Each substrate and site has a specific shape and in many cases only certain molecules will be allowed to fit into the site, which may be on an enzyme, the surface of a cell or at a nerve ending (see Chapter 11 ‘Amino acids and proteins’, p. 85 and Chapter 19 ‘Pharmacodynamics: how drugs elicit a physiological effect’, p. 137).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree