Chapter 4 Bonds continued
Chapter 3 introduced bond formation. This chapter looks at more complex bonds, where several electrons are shared to form double or triple bonds. These bonds are relevant to the type of chemistry you will encounter in clinical practice.
Double Bonds
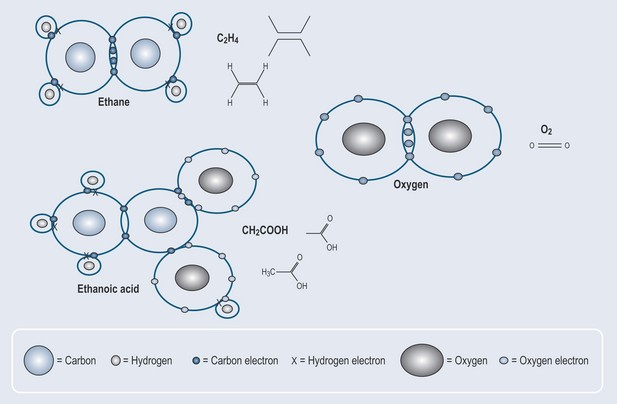
Carbon and oxygen atoms can form a double bond. Figure 4.1 gives the examples of double bonds found in pharmacology. The chemical shorthand for these structures is shown alongside the detailed diagrams of electron distribution.
Why is a double bond significant?
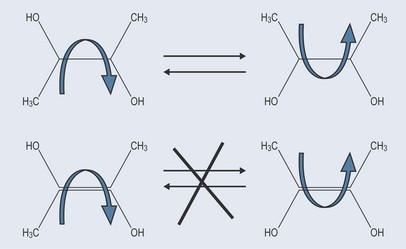
When carbon atoms are joined by a single bond, rotation can easily occur around the bond. When there is a double bond this mobility is not possible (Figure 4.2). The structure of the double bond will not allow this rotation and rigidly fixes the shape of that molecule around the double bond. This inability to change orientation is very important in pharmacology. For example, chemically active compounds might have the same molecular formula but different chemical reactions as the components of the compounds orientated differently in space. The different actions of cis and trans fatty acids on the body are important examples of geometrical isomerism (see Chapter 6 ‘Isomers’, p. 38 and Chapter 10 ‘Lipids’, p. 74).
Triple Bonds
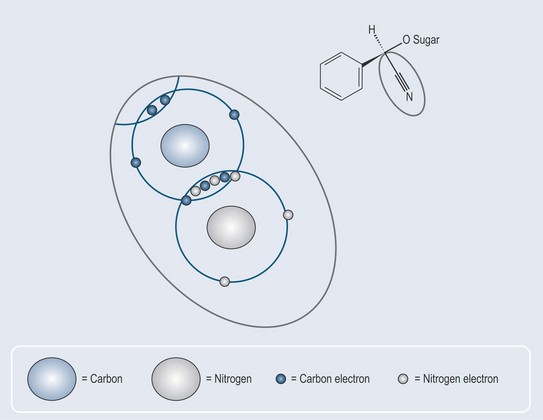
Triple bonds (Figure 4.3) occur in nature in cyanogenic glycosides (see Chapter 24 ‘Glycosides’, p. 183) they are not as common as single or double bonds.
Aromatic Rings
What happens when alternating double and single bonds join to form a ring? If this occurs a very interesting structure called an aromatic ring forms. The bonds in an aromatic ring are particularly interesting.
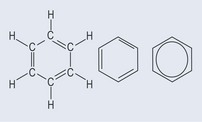
The basis of aromatic ring is benzene (Figure 4.4). In theory, benzene has three double bonds, but a molecule that is not a ring structure and has double bonds reacts very differently chemically to benzene. There are also other quibbles with bond lengths and shape, which are outside the scope of the book.
The chemical assumption that has to be made is that the electrons associated with the benzene ring are, more or less, evenly shared around the ring, so it is impossible to tell where the double and single bonds are. Thus, although the benzene ring can be thought of as a hexagon, and although the bonds are shown for the purposes of presenting a standard structure, there are, in effect, no alternating double and single bonds. In many cases, the ring is shown as the far right notation in Figure 4.4, which indicates the sharing of the electrons. This sharing gives the structure unique qualities when it comes to chemical reactions, because the hydrogen atoms around the ring can be substituted and replaced by a new functional group (see Figure 5.1, p. 30) while the benzene ring stays intact rather than breaking open and changing shape.